
Polymerization of ethene gives poly(ethane)
How does the bonding between carbon atoms in poly(ethane) compare with that in ethane?
A.Longer and stronger in poly(ethene)
B.Longer and weaker in poly(ethene)
C.Shorter and stronger in poly(ethene)
D.Shorter and weaker in poly(ethene)
Answer
521.1k+ views
Hint: Polymerization is a process in monomers, combines chemically to produce a very large chainlike or network molecule, called a polymer. The monomer molecules may be similar or they may represent two, three, or more different compounds. The more the branching of the compared stronger the polymer will be.
Complete answer:
Structure of polyethylene is something like below:
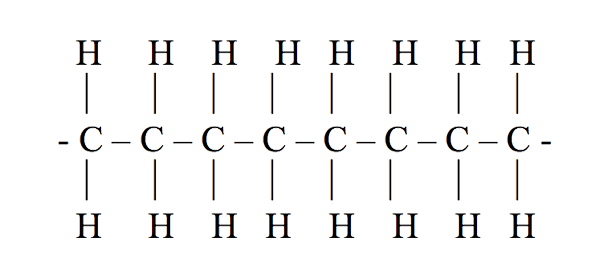
Where ethane is the monomer and numerous of them combine to form polyethylene. This poly(ethane) is also known as polyethylene.
Polyethylene is just a long chain of carbons, with two hydrogens attached to each carbon atom. Here the number of ethane monomers is not fixed; it can be in any amount, maybe hundreds or thousands. Sometimes, instead of hydrogens in the chain, there will be some branches of polyethylene or long chains of polyethylene attached to them. The structure that will be formed by that is called branched polyethylene or low-density polyethylene (LDPE). The structure above is a single long chain so it is linear polyethylene.
When we have a long chain with a single bond the bonding is usually weaker than the small monomer molecule. When you try to break the long chain it is easier to break but when you try to break the shorter one it will take time to break. The chain is linear and long so it will be weaker than the ethane molecule.
Correct option: B. Longer and weaker in poly(ethene).
Note:
You can imagine the above case in that way, suppose you have a very long thin thread and a short thin thread both are made up of the same material. When you try to break the long thread it is easier to break but when you try to break the shorter one it will take time to break that.
Complete answer:
Structure of polyethylene is something like below:

Where ethane is the monomer and numerous of them combine to form polyethylene. This poly(ethane) is also known as polyethylene.
Polyethylene is just a long chain of carbons, with two hydrogens attached to each carbon atom. Here the number of ethane monomers is not fixed; it can be in any amount, maybe hundreds or thousands. Sometimes, instead of hydrogens in the chain, there will be some branches of polyethylene or long chains of polyethylene attached to them. The structure that will be formed by that is called branched polyethylene or low-density polyethylene (LDPE). The structure above is a single long chain so it is linear polyethylene.
When we have a long chain with a single bond the bonding is usually weaker than the small monomer molecule. When you try to break the long chain it is easier to break but when you try to break the shorter one it will take time to break. The chain is linear and long so it will be weaker than the ethane molecule.
Correct option: B. Longer and weaker in poly(ethene).
Note:
You can imagine the above case in that way, suppose you have a very long thin thread and a short thin thread both are made up of the same material. When you try to break the long thread it is easier to break but when you try to break the shorter one it will take time to break that.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




