
Polymer used in bullet-proof glass is _________.
A.Nomex
B.Lexan
C.PMMA
D.Kevlar
Answer
590.4k+ views
Hint: Bullet-proof glass is a strong and optically transparent material which is used in the resistance of penetration of projectiles like gun bullets, bomb explosions etc. It is almost impenetrable glass and it is also known as armor glass.
Complete step by step answer:
The material used in making bullet proof glass should be hard, tough, durable and optically transparent. The only polymer that matches these qualities is thermosetting polymer containing carbonate groups or polycarbonates.
All the properties and qualities required by bullet-proof glass are present in Lexan which is also known as laminated polycarbonate, so Lexan is the polymer which is used in bullet-proof glasses.
Structure of Lexan
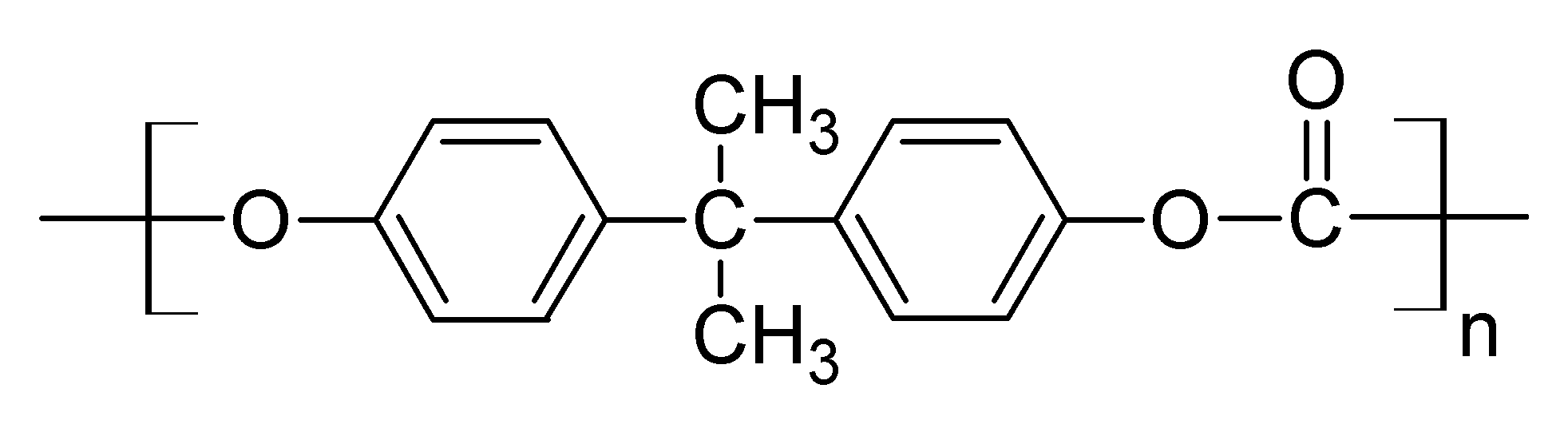
Lexan is produced by reacting a chemical compound Bisphenol A with carbonyl chloride (phosgene), the base resin that makes up the Laxen is produced. This resin is cut into various dimensions. This is the process of formation of resins.
Formation reaction of Lexan:
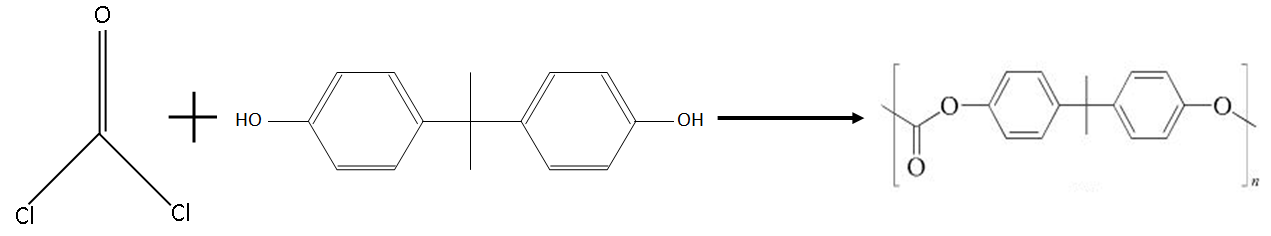
Note:
The reaction involved in the formation of Lexan is a polymerization reaction or we can say condensation polymerization. This condensation process occurs in this reaction because this reaction occurs between an acid and alcohol.
Complete step by step answer:
The material used in making bullet proof glass should be hard, tough, durable and optically transparent. The only polymer that matches these qualities is thermosetting polymer containing carbonate groups or polycarbonates.
All the properties and qualities required by bullet-proof glass are present in Lexan which is also known as laminated polycarbonate, so Lexan is the polymer which is used in bullet-proof glasses.
Structure of Lexan

Lexan is produced by reacting a chemical compound Bisphenol A with carbonyl chloride (phosgene), the base resin that makes up the Laxen is produced. This resin is cut into various dimensions. This is the process of formation of resins.
Formation reaction of Lexan:

Note:
The reaction involved in the formation of Lexan is a polymerization reaction or we can say condensation polymerization. This condensation process occurs in this reaction because this reaction occurs between an acid and alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




