
Phenolphthalein gives a pink colour in alkaline medium due to the fact that:
Answer
590.4k+ views
Hint: An attempt to this question can be done by drawing the structure of phenolphthalein and trying to write the chemical reaction between phenolphthalein and an acid like HCl and see if there are ions being released.
Complete answer:
Phenolphthalein is widely used as an indicator in acid-base titrations.
Now we will draw the structure of phenolphthalein:
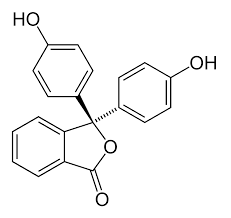
From the above structure we come to know that phenolphthalein has acidic groups ( hydroxyl groups). These groups do not react with the acid added to the solution as both are acidic in nature and hence no ions are formed. This is the reason why the colour of phenolphthalein does not change when an acid is added to it.
Therefore, the correct answer is option (D).
Note:
Phenolphthalein solution turns pink in the presence of a base. This is because the hydroxide ions react with the acidic group present and lead to the formation of ions. These ions impart color to the solution. This is how phenolphthalein solution acts as an acid-base indicator in titrations. We say that the titration has reached the neutralization point when the solution in the flask starts turning pink indicating that the acid has been neutralized.
The reaction between phenolphthalein and a base is given below:
Complete answer:
Phenolphthalein is widely used as an indicator in acid-base titrations.
Now we will draw the structure of phenolphthalein:

From the above structure we come to know that phenolphthalein has acidic groups ( hydroxyl groups). These groups do not react with the acid added to the solution as both are acidic in nature and hence no ions are formed. This is the reason why the colour of phenolphthalein does not change when an acid is added to it.
Therefore, the correct answer is option (D).
Note:
Phenolphthalein solution turns pink in the presence of a base. This is because the hydroxide ions react with the acidic group present and lead to the formation of ions. These ions impart color to the solution. This is how phenolphthalein solution acts as an acid-base indicator in titrations. We say that the titration has reached the neutralization point when the solution in the flask starts turning pink indicating that the acid has been neutralized.
The reaction between phenolphthalein and a base is given below:
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




