
Phenol reacts with bromine in at low temperature to give:
A. m-bromophenol
B. p-bromophenol
C. o and p-bromophenol
D. $ 2,4,6 - tribromophenol $
Answer
546.9k+ views
Hint :When phenol is treated with bromine at low temperature in the presence of $ C{S_2} $ (non-polar solvent) gives mono bromophenol which includes p-bromophenol and o-bromophenol.
Complete Step By Step Answer:
In the given question, phenol reacts with bromine in $ C{S_2} $ at the low temperature that is $ 273K $ .
Here it will give two compounds at the ortho and the para position of the phenol.
Phenol is a benzene ring with OH at the top. This will also give another product which is $ HBr $ .
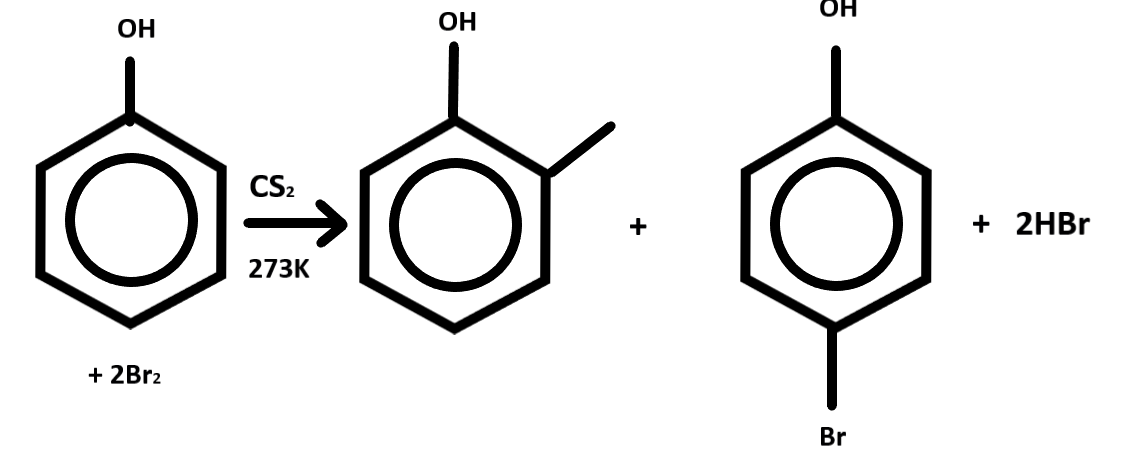
While going through the product this will go through electrophilic substitution, $ C{S_2} $ is the non-polar compound which acts as the catalyst here. We get one major product and one minor product.
p-bromophenol is the major product and o-bromophenol is the minor product. Here bromine is added on ortho and para directors are only as they are a strongly activating group. So the final product we get from the reaction of phenol with bromine is o and p-bromophenol.
Note :
Electrophilic substitution reactions are the chemical reaction in which a functional group is displaced by an electrophile in a compound. This one will involve the generation of an electrophile, after that there is an intermediate formation of a carbonate. At the end, the most important step is the removal of the proton from the intermediate. It is of two types that is aliphatic reactions and aromatic reactions. Since bromine is the halogen so it’s the halogenation electrophilic reaction.
Complete Step By Step Answer:
In the given question, phenol reacts with bromine in $ C{S_2} $ at the low temperature that is $ 273K $ .
Here it will give two compounds at the ortho and the para position of the phenol.
Phenol is a benzene ring with OH at the top. This will also give another product which is $ HBr $ .

While going through the product this will go through electrophilic substitution, $ C{S_2} $ is the non-polar compound which acts as the catalyst here. We get one major product and one minor product.
p-bromophenol is the major product and o-bromophenol is the minor product. Here bromine is added on ortho and para directors are only as they are a strongly activating group. So the final product we get from the reaction of phenol with bromine is o and p-bromophenol.
Note :
Electrophilic substitution reactions are the chemical reaction in which a functional group is displaced by an electrophile in a compound. This one will involve the generation of an electrophile, after that there is an intermediate formation of a carbonate. At the end, the most important step is the removal of the proton from the intermediate. It is of two types that is aliphatic reactions and aromatic reactions. Since bromine is the halogen so it’s the halogenation electrophilic reaction.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




