
Paracetamol is:
a.) methyl salicylate
b.) phenyl salicylate
c.) N-acetyl p-aminophenol
d.) acetyl salicylic acid
Answer
590.4k+ views
Hint: Paracetamol is used as an antipyretic drug. The molecule of paracetamol consists of a benzene ring with two substitutions at 1st and 4th position. The one position is occupied by hydroxyl group while the para position is occupied by acetamide group. The 4-amino phenol is obtained on amide hydrolysis of paracetamol.
Complete step by step answer :
Before jumping into the solution, let us first understand what paracetamol is. After this, we will be able to find the correct answer.
Paracetamol is a common name in our house. It is the important constituent of our first-aid kit. And this is the tablet whose function anyone can tell.
So, this is a drug used to relieve pain and reduce fever. It is used in case of headache, backache, fever, common cold and during mild pain in arthritis.
When we see the structure of paracetamol, it consists of one benzene ring which is substituted by hydroxyl group at one position and at its para position by nitrogen atom of ethanamide group. These both groups are activating in nature and thus activate the ring towards electrophilic aromatic substitution. Further, the structure formed by these two substitutions is highly conjugated in nature because the lone pair of oxygen, pi electrons of benzene and lone pair on nitrogen all participate in the conjugation.
The structure of paracetamol is as-
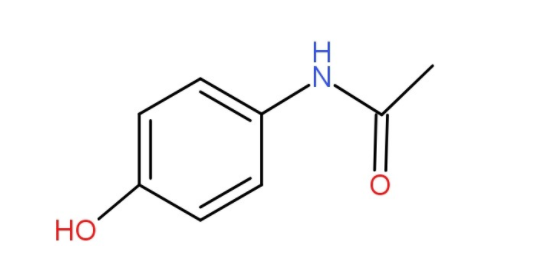
Thus, by observing the structure, we can say that the molecule has one benzene ring and one hydroxyl group. So, it is phenol. Further, the ring is substituted at para position by acetamide.
So, the complete name could be N-acetyl p-aminophenol.
Thus, the option c.) will be the correct answer.
Note: Paracetamol is part of aniline analgesic drugs.
The conjugation present in paracetamol reduces the basicity of oxygen atoms and nitrogen atoms. The hydroxyl group however becomes more acidic because the negative charge can delocalise by phenoxide ion.
Complete step by step answer :
Before jumping into the solution, let us first understand what paracetamol is. After this, we will be able to find the correct answer.
Paracetamol is a common name in our house. It is the important constituent of our first-aid kit. And this is the tablet whose function anyone can tell.
So, this is a drug used to relieve pain and reduce fever. It is used in case of headache, backache, fever, common cold and during mild pain in arthritis.
When we see the structure of paracetamol, it consists of one benzene ring which is substituted by hydroxyl group at one position and at its para position by nitrogen atom of ethanamide group. These both groups are activating in nature and thus activate the ring towards electrophilic aromatic substitution. Further, the structure formed by these two substitutions is highly conjugated in nature because the lone pair of oxygen, pi electrons of benzene and lone pair on nitrogen all participate in the conjugation.
The structure of paracetamol is as-

Thus, by observing the structure, we can say that the molecule has one benzene ring and one hydroxyl group. So, it is phenol. Further, the ring is substituted at para position by acetamide.
So, the complete name could be N-acetyl p-aminophenol.
Thus, the option c.) will be the correct answer.
Note: Paracetamol is part of aniline analgesic drugs.
The conjugation present in paracetamol reduces the basicity of oxygen atoms and nitrogen atoms. The hydroxyl group however becomes more acidic because the negative charge can delocalise by phenoxide ion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




