
P is
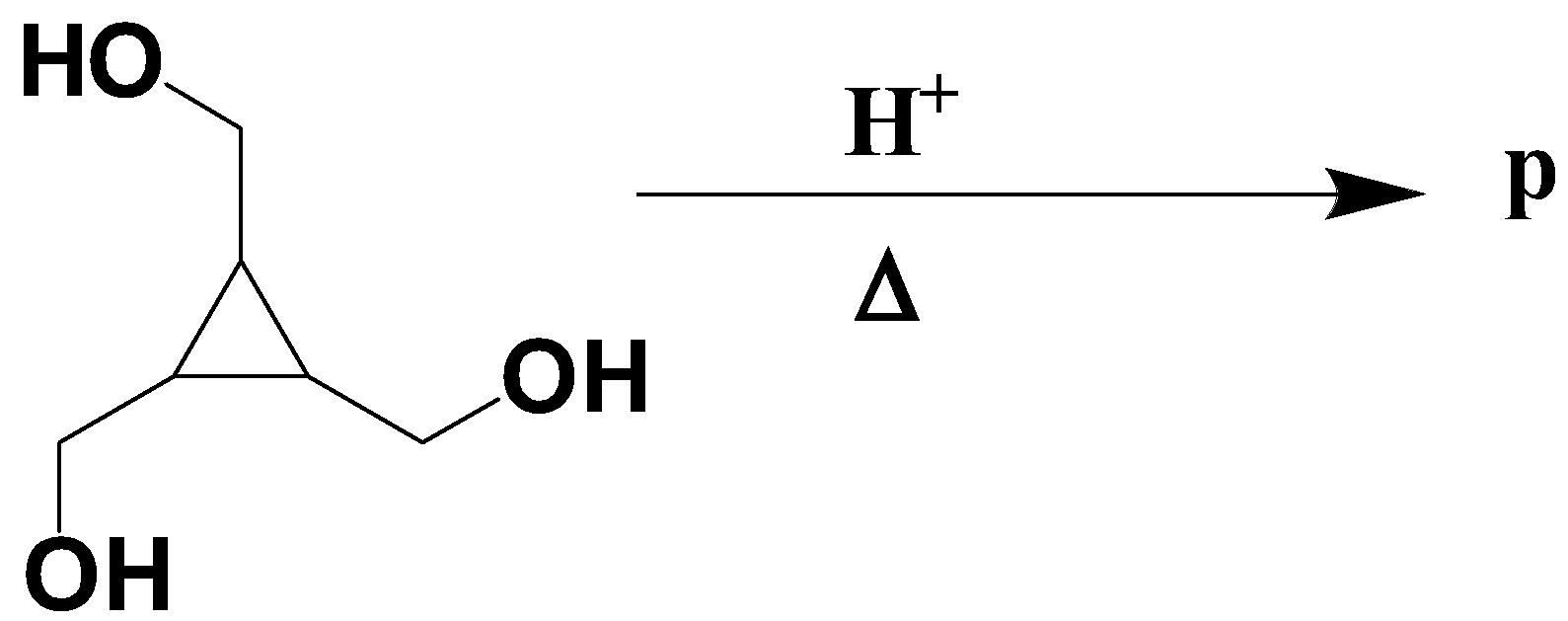
Info there is interconversion between the given compounds
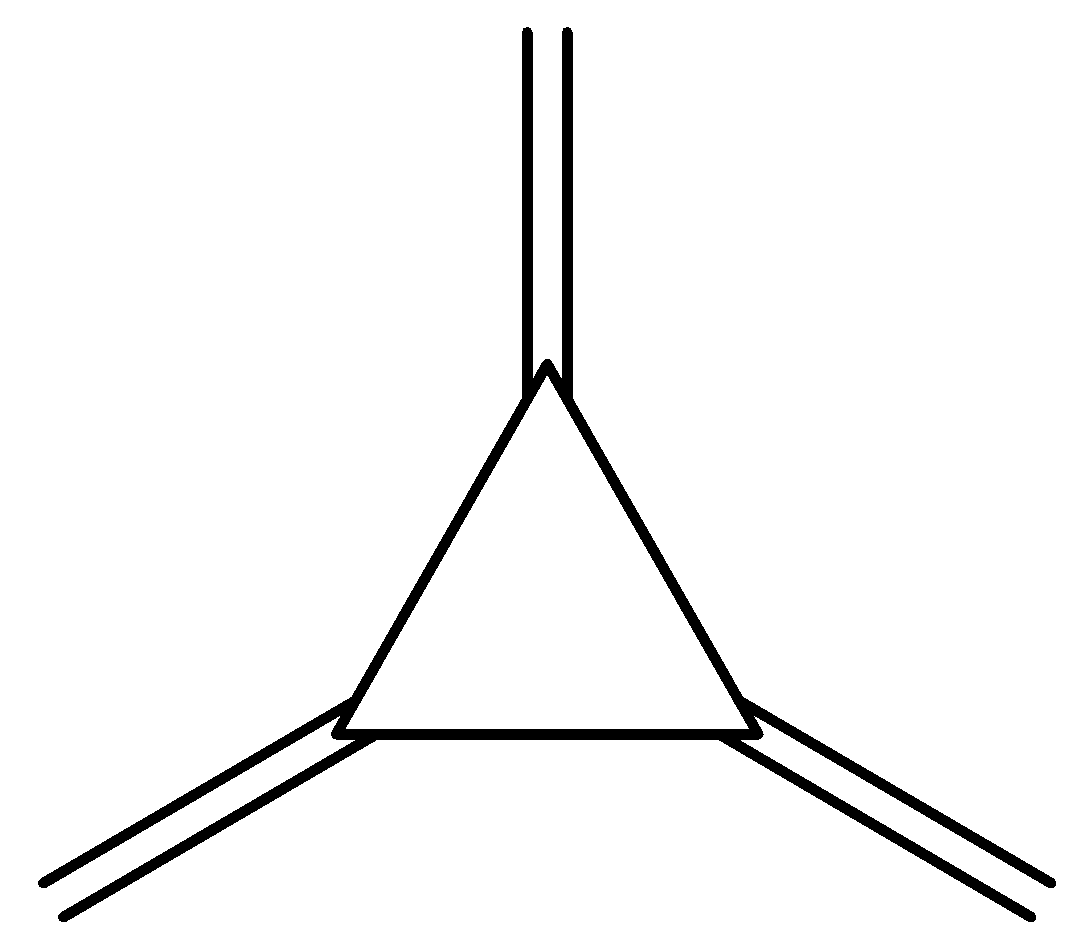
A.
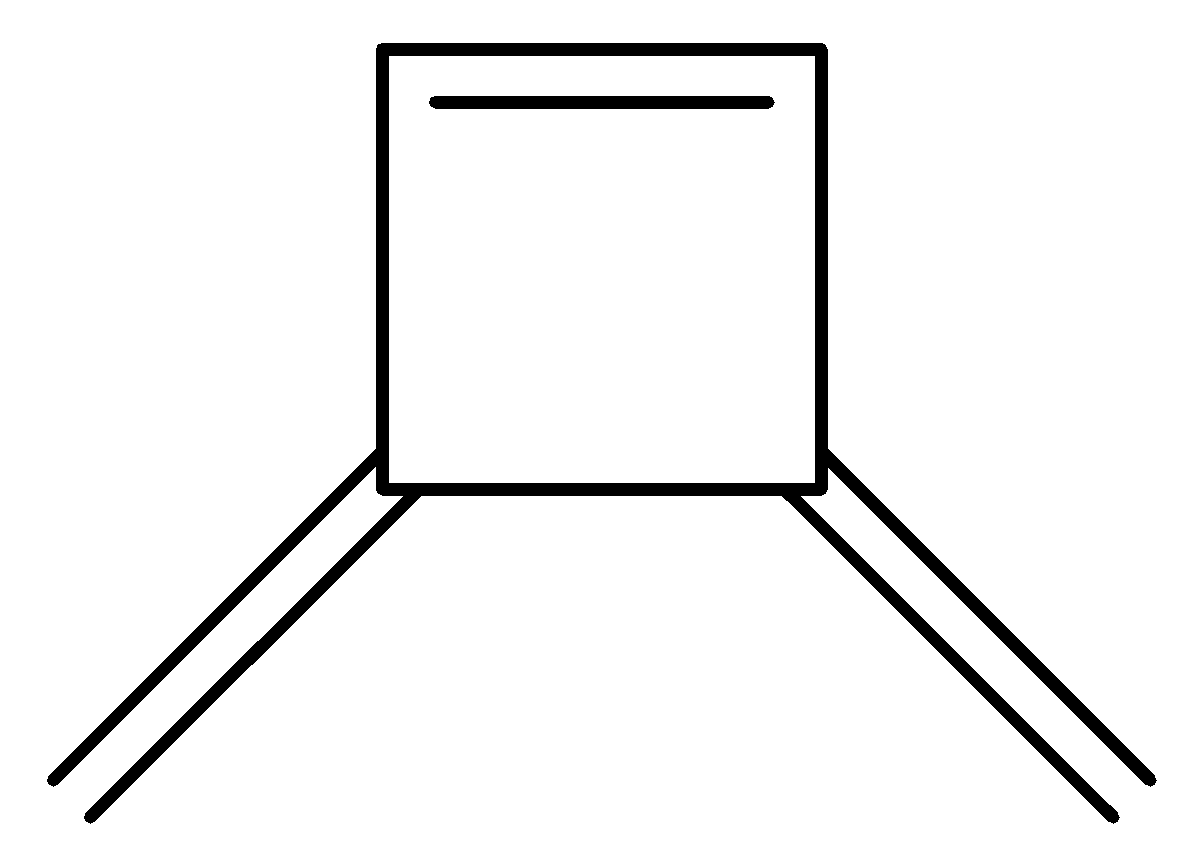
B.
C.
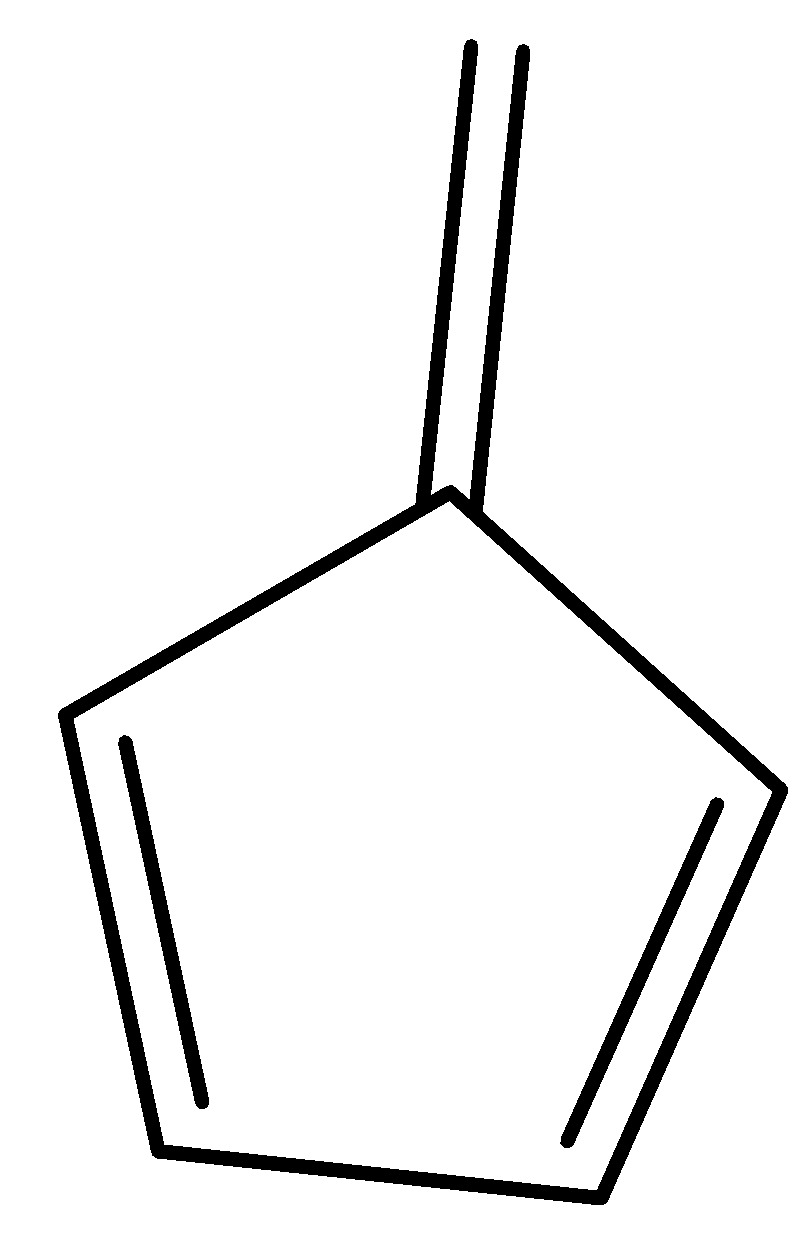
D.
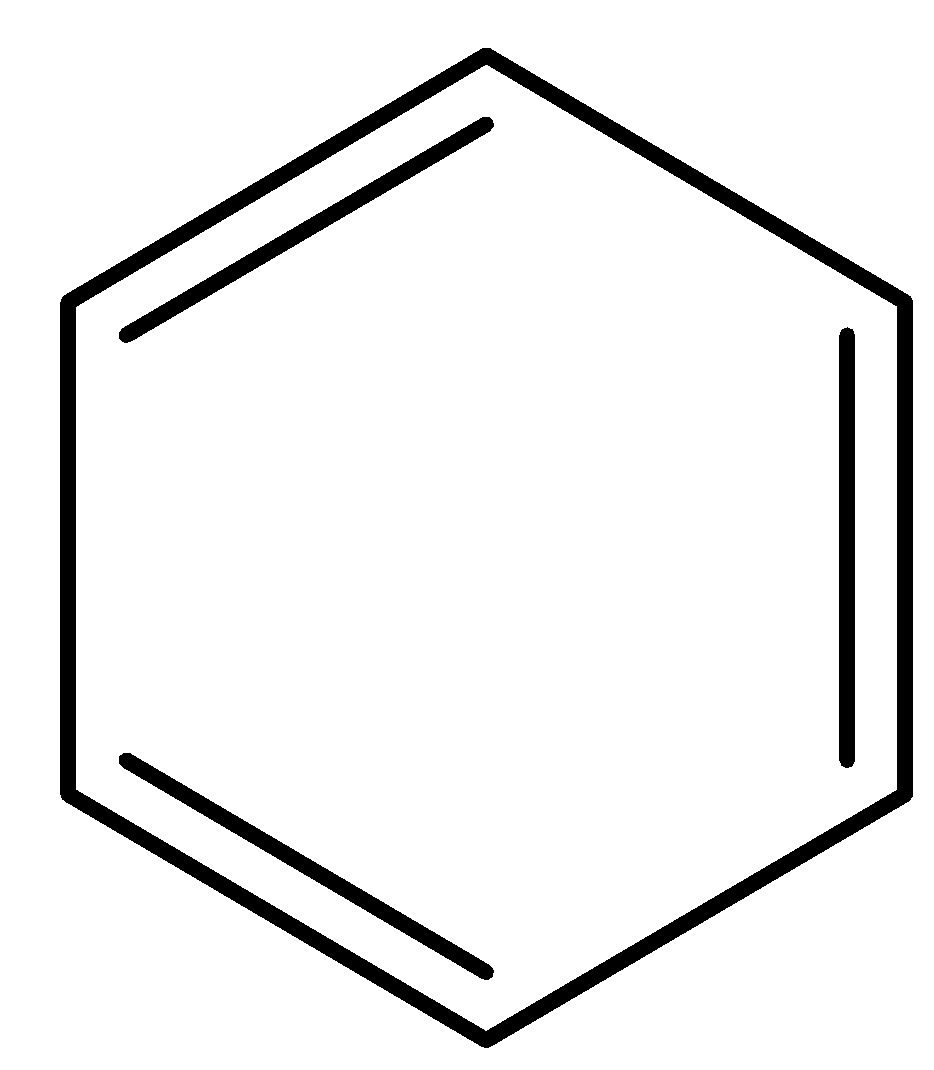






Answer
560.7k+ views
Hint: In the chemistry carbon is a very versatile element which has a tetravalent structure which means it can attach to mostly four elements at a time, but it also shows a wide variety of bond structure ranging from the single bond, double bond and triple bond. All these bonds take different types of strengths and exist with different electrons which are in participation.
Complete step by step answer:
Carbon is a very versatile element and showcases a wide range of bond types ranging from single to triple. All these bonds exist but the properties of these bonds are very different. The carbon is said to be most stable in its quaternary state which means the carbon is the most stable when it is surrounded by four other elements. This is possible only when all the elements are connected to the central atom through the single bond.
Upon the interaction with hydrogen ions, the alcohol group in the compound leaves the compound to bond with the hydrogen atom to form water molecules which will result in the formation of the following compound.
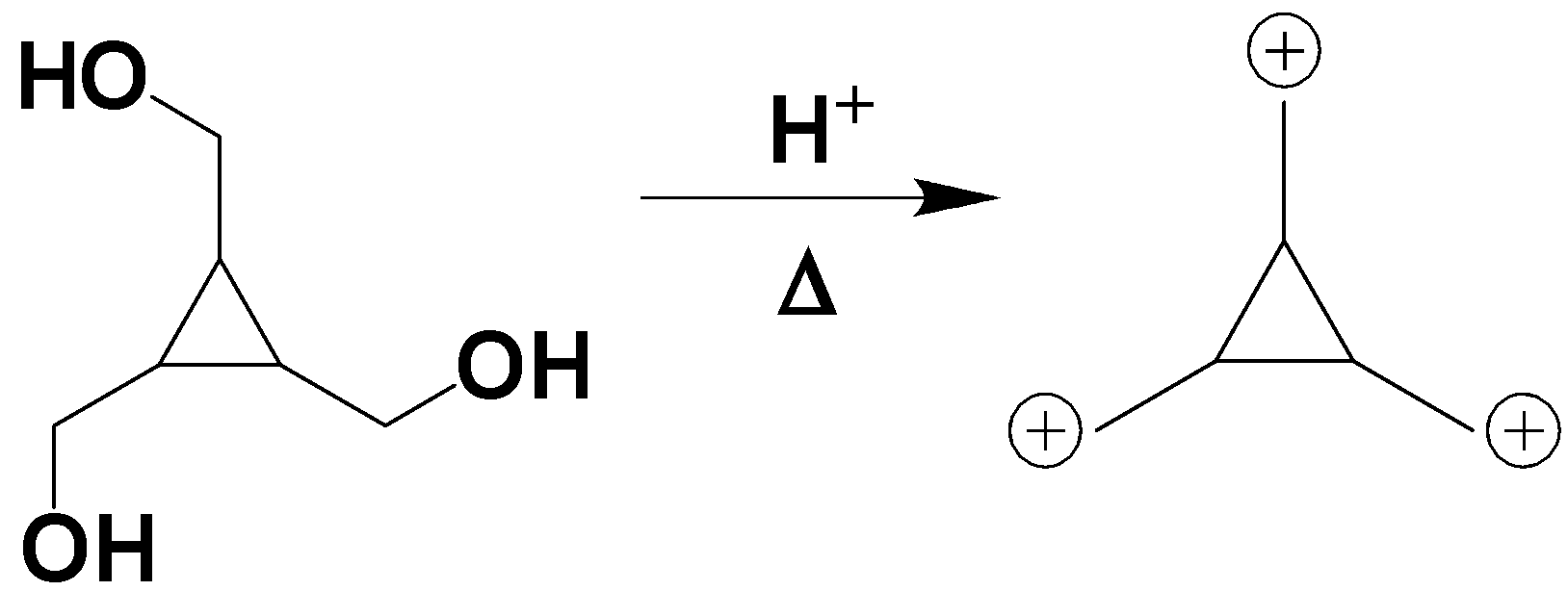
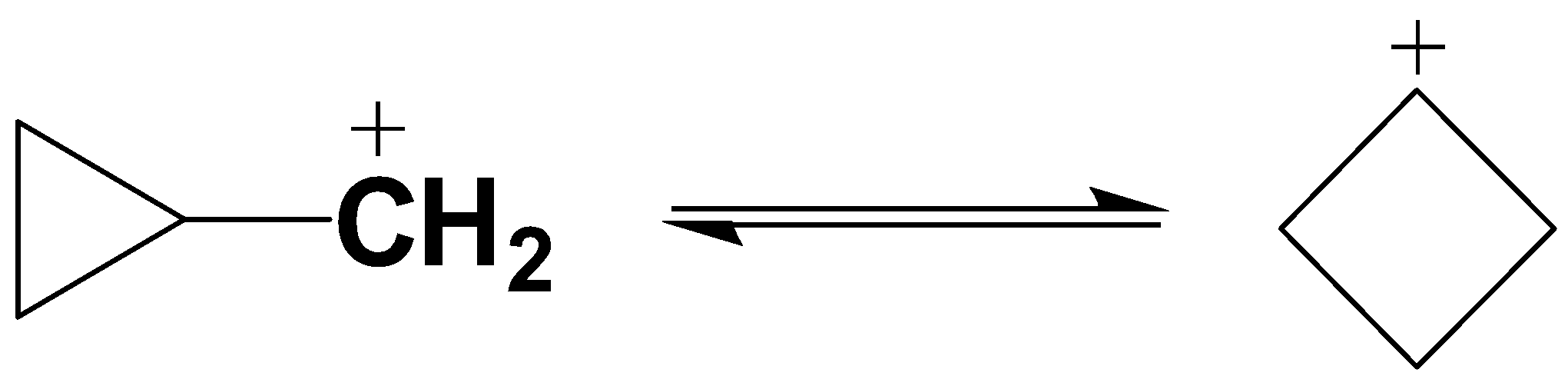
As given in the question the following compound will rearrange and interconvert to the following compound due to the resonance of the electron pair.
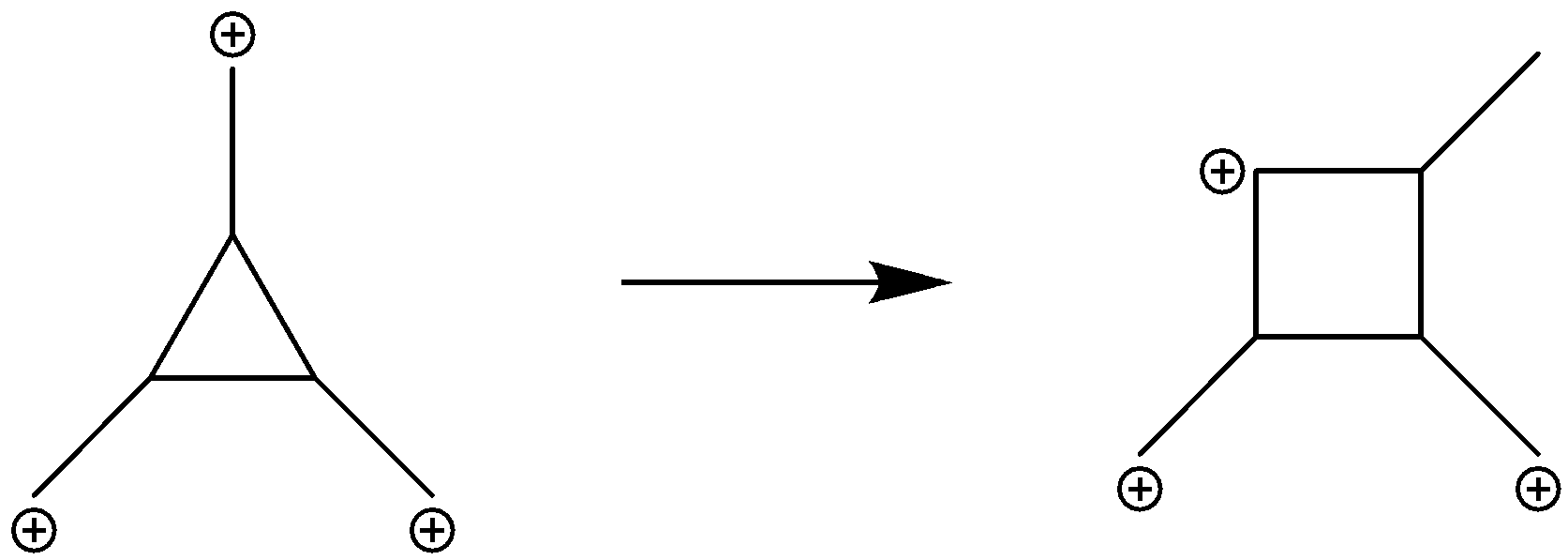
The further rearrangements and rearrangements will be as depicted in the following reactions. Form
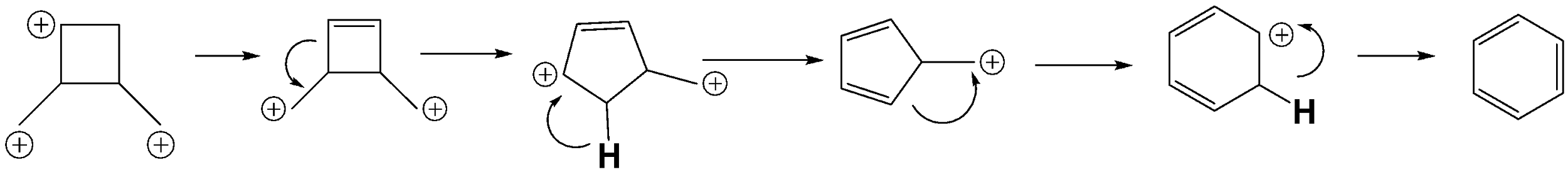
So the compound rearranges to form benzene
So, the correct answer is Option D.
Note: Various effects operate in the chemical structure of the compounds. The range from inductive effect, resonance effect, mesomeric effect etc. here +M is the mesomeric effect. These effects can be positive or negative based on the substituent groups which are attached to the main compound.
Complete step by step answer:
Carbon is a very versatile element and showcases a wide range of bond types ranging from single to triple. All these bonds exist but the properties of these bonds are very different. The carbon is said to be most stable in its quaternary state which means the carbon is the most stable when it is surrounded by four other elements. This is possible only when all the elements are connected to the central atom through the single bond.
Upon the interaction with hydrogen ions, the alcohol group in the compound leaves the compound to bond with the hydrogen atom to form water molecules which will result in the formation of the following compound.

As given in the question the following compound will rearrange and interconvert to the following compound due to the resonance of the electron pair.

The further rearrangements and rearrangements will be as depicted in the following reactions. Form

So the compound rearranges to form benzene
So, the correct answer is Option D.
Note: Various effects operate in the chemical structure of the compounds. The range from inductive effect, resonance effect, mesomeric effect etc. here +M is the mesomeric effect. These effects can be positive or negative based on the substituent groups which are attached to the main compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




