
Oxidation state of \[S\] in sodium tetrathionate is
A. \[+5,0,0,+5\]
B. \[+6,+6,0\]
C. \[+4,+2,+5\]
D. \[+2,0,+2,+3\]
Answer
573.6k+ views
Hint: The structure of sodium tetrathionate contains two atoms of sodium and four atoms of sulphur along with six atoms of oxygen attached to one another.
The molecule has overall charge zero which indicates that all the oxidation states of individual atoms involved in the molecule balances the charges possessed by each other.
Complete step by step answer:
Oxidation state which is also sometimes called oxidation number is the total number of electrons possessed by an atom which are obtained either by the loss or gain of electrons, in order to form a chemical bond along with another atom. Every atom which is involved in the reaction of reduction or oxidation has been assigned some oxidation state which indicates the ability of that atom to accept, share or donate electrons.
Consider the molecule given in the question. The structure of the molecule is shown below.
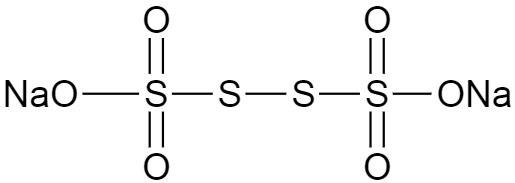
As we can see, there are four sulphur atoms in one molecule of sodium tetrathionate, and the overall charge in the molecule is zero. Now we know that general oxidation state of oxygen is $-2$ and that of sodium is $+1$, so if we calculate the total oxidation state, without the oxidation number of sulphur atoms we get
$(2\times 6)-2=10$
Where, the total number of oxygen atoms are $6$ and that of sodium atoms is $2$.
So the sulphur atoms which are present at the middle of the structure will have zero oxidation state, and the terminal sulphur atoms will have $+5$ oxidation state each.
So, the correct answer is Option A.
Note: The sodium tetrathionate can be produced by oxidation of sodium thiosulphate, in presence of iodine. The reaction attains completion by discoloration of the solution. This change in colour is due to the change in oxidation state of the sulphur present in thiosulphate.Sodium tetrathionate is a colourless, water soluble solid, which usually exists in white powder form.
The molecule has overall charge zero which indicates that all the oxidation states of individual atoms involved in the molecule balances the charges possessed by each other.
Complete step by step answer:
Oxidation state which is also sometimes called oxidation number is the total number of electrons possessed by an atom which are obtained either by the loss or gain of electrons, in order to form a chemical bond along with another atom. Every atom which is involved in the reaction of reduction or oxidation has been assigned some oxidation state which indicates the ability of that atom to accept, share or donate electrons.
Consider the molecule given in the question. The structure of the molecule is shown below.
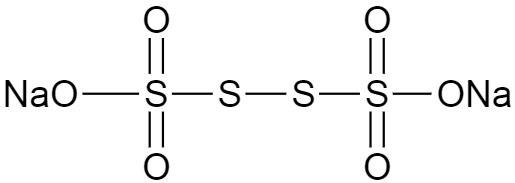
As we can see, there are four sulphur atoms in one molecule of sodium tetrathionate, and the overall charge in the molecule is zero. Now we know that general oxidation state of oxygen is $-2$ and that of sodium is $+1$, so if we calculate the total oxidation state, without the oxidation number of sulphur atoms we get
$(2\times 6)-2=10$
Where, the total number of oxygen atoms are $6$ and that of sodium atoms is $2$.
So the sulphur atoms which are present at the middle of the structure will have zero oxidation state, and the terminal sulphur atoms will have $+5$ oxidation state each.
So, the correct answer is Option A.
Note: The sodium tetrathionate can be produced by oxidation of sodium thiosulphate, in presence of iodine. The reaction attains completion by discoloration of the solution. This change in colour is due to the change in oxidation state of the sulphur present in thiosulphate.Sodium tetrathionate is a colourless, water soluble solid, which usually exists in white powder form.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




