
Oxidation of toluene to benzaldehyde by the use of chromyl chloride is called?
A) Wurtz reaction
B) Etard’s reaction
C) Fittig reaction
D) Rosenmund’s reaction
Answer
577.2k+ views
Hint:We know that benzaldehyde is an organic compound which consists of a benzene ring to which a formyl $\left( { - {\text{CHO}}} \right)$ group is attached. Chromyl chloride behaves as a weak acid in a nonpolar solvent. The methyl group of toluene gets partially oxidised by the chromyl chloride in this reaction.
Complete answer
The conversion of toluene to benzaldehyde by the use of chromyl chloride is an oxidation reaction. This reaction is known as Etard’s reaction. In this reaction, the compounds having at least one methyl group bonded to the benzene ring are required. The methyl group gets oxidised and converts to an aldehyde group. The oxidation of the methyl group is done using a weak oxidising agent. Thus, chromyl chloride which is a weak oxidising agent is used.
The overall mechanism of the reaction is as follows:
Step 1: The hydrogen from the methyl group of toluene is abstracted by the chromyl chloride. The chromyl chloride forms a bond with the benzene ring. This complex is known as Etard’s complex.
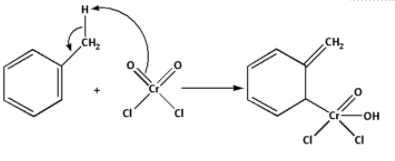
Step 2: The Etard’s complex formed in the first step undergoes rearrangement. The complex undergoes a 2,3-sigmatropic rearrangement. Thus, a rearrangement product is formed.
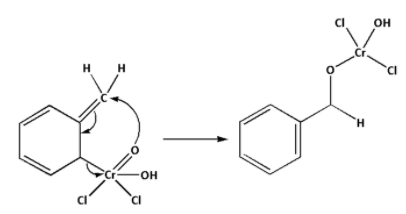
Step 3: The product formed in the above step is the rearrangement product. and are eliminated from the rearrangement product. This leads to the formation of benzaldehyde.
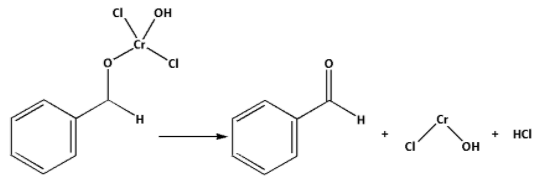
Thus, oxidation of toluene to benzaldehyde by the use of chromyl chloride is called Etard’s reaction.
Thus, the correct answer is option (B) Etard’s reaction.
Note:
i) Wurtz-Fittig reaction: In this reaction, two alkyl halides react with sodium metal in dry ether and produce a higher alkane.
ii) Rosenmund’s reaction: In this reaction, an acyl chloride is hydrogenated over catalyst, palladium on barium sulphate to produce aldehyde.
Complete answer
The conversion of toluene to benzaldehyde by the use of chromyl chloride is an oxidation reaction. This reaction is known as Etard’s reaction. In this reaction, the compounds having at least one methyl group bonded to the benzene ring are required. The methyl group gets oxidised and converts to an aldehyde group. The oxidation of the methyl group is done using a weak oxidising agent. Thus, chromyl chloride which is a weak oxidising agent is used.
The overall mechanism of the reaction is as follows:
Step 1: The hydrogen from the methyl group of toluene is abstracted by the chromyl chloride. The chromyl chloride forms a bond with the benzene ring. This complex is known as Etard’s complex.

Step 2: The Etard’s complex formed in the first step undergoes rearrangement. The complex undergoes a 2,3-sigmatropic rearrangement. Thus, a rearrangement product is formed.

Step 3: The product formed in the above step is the rearrangement product. and are eliminated from the rearrangement product. This leads to the formation of benzaldehyde.

Thus, oxidation of toluene to benzaldehyde by the use of chromyl chloride is called Etard’s reaction.
Thus, the correct answer is option (B) Etard’s reaction.
Note:
i) Wurtz-Fittig reaction: In this reaction, two alkyl halides react with sodium metal in dry ether and produce a higher alkane.
ii) Rosenmund’s reaction: In this reaction, an acyl chloride is hydrogenated over catalyst, palladium on barium sulphate to produce aldehyde.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




