
Oxidation of aniline with acidified potassium dichromate gives:
A) p-benzoquinone
B) Benzoic acid
C) Benzaldehyde
D) Benzyl alcohol
Answer
598.2k+ views
Hint: Simplest aromatic amine, aniline undergoes the oxidation with acidified potassium dichromate to get the oxidised product through the 4-aminophenol and quinoneimine as the intermediates. The dichromate in acid produces the oxygen atom which oxidises the aniline to form a 1, 4-benzoquinone.
Complete answer:
Amines in which the nitrogen atom is directly bonded to the one or more aromatic rings are called the aromatic amine. Aniline is an example of primary $({{1}^{0}})$ aromatic amine.
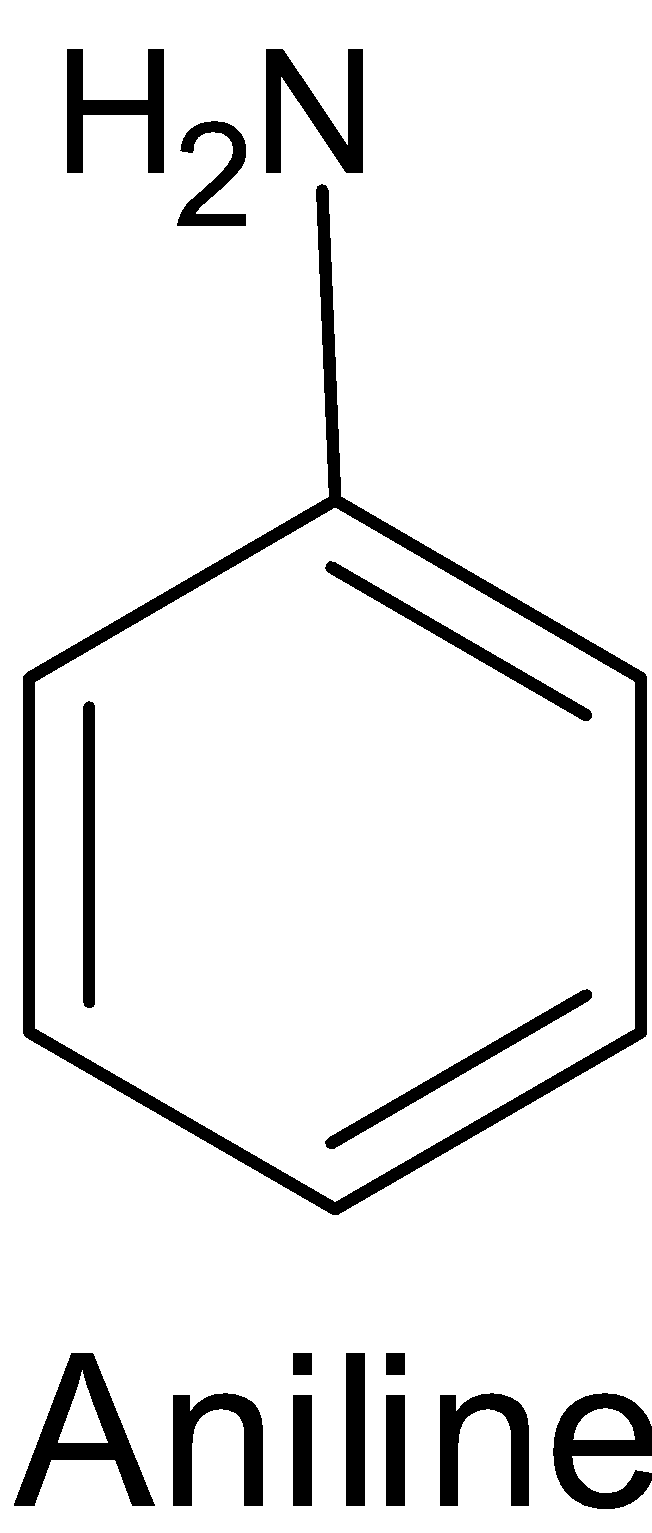
Aromatic amines like the aniline $\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{-N}{{\text{H}}_{\text{2}}}\text{)}$ undergo various type of reactions. One such reaction is oxidation.
An oxidation reaction is defined as the addition of oxygen to an organic compound. It is also a reaction of the removal of hydrogen or removal of an electron.
Oxidation of amines gives different products depending on the nature of amine and oxidizing agent. Amines undergo oxidation by a powerful oxidizing agents such as $\text{KMn}{{\text{O}}_{\text{4}}}$ hydrogen peroxide,${{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$, etc.
In the case of aromatic amine, because of the high electron density on the benzene ring they are readily oxidized on exposure to air or oxidizing agents.
The amine undergoes the oxidation reaction in presence of acidified potassium dichromate. Potassium dichromate ${{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ is a very strong oxidizing agent that readily adds the oxygen atom in the compound. In presence of dil.${{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}$, the ${{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ gives the three atoms of oxygen as follows.
${{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\text{+4}{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\to {{\text{K}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{+Cr2(S}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}\text{+3}\left[ \text{O} \right]\text{+2}{{\text{H}}_{\text{2}}}\text{O}$
These three nascent oxygen atoms oxidized then aniline effectively.
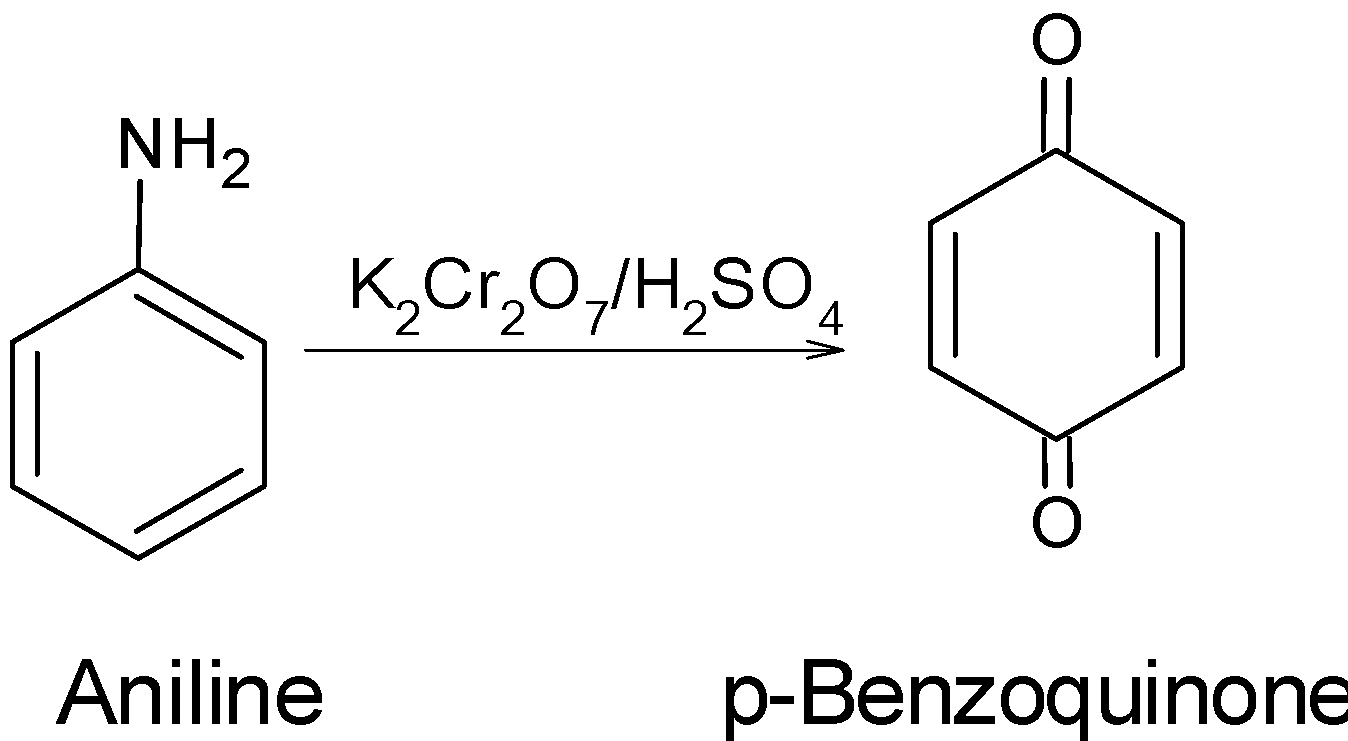
Hence, (A) is the correct option.
Additional information:
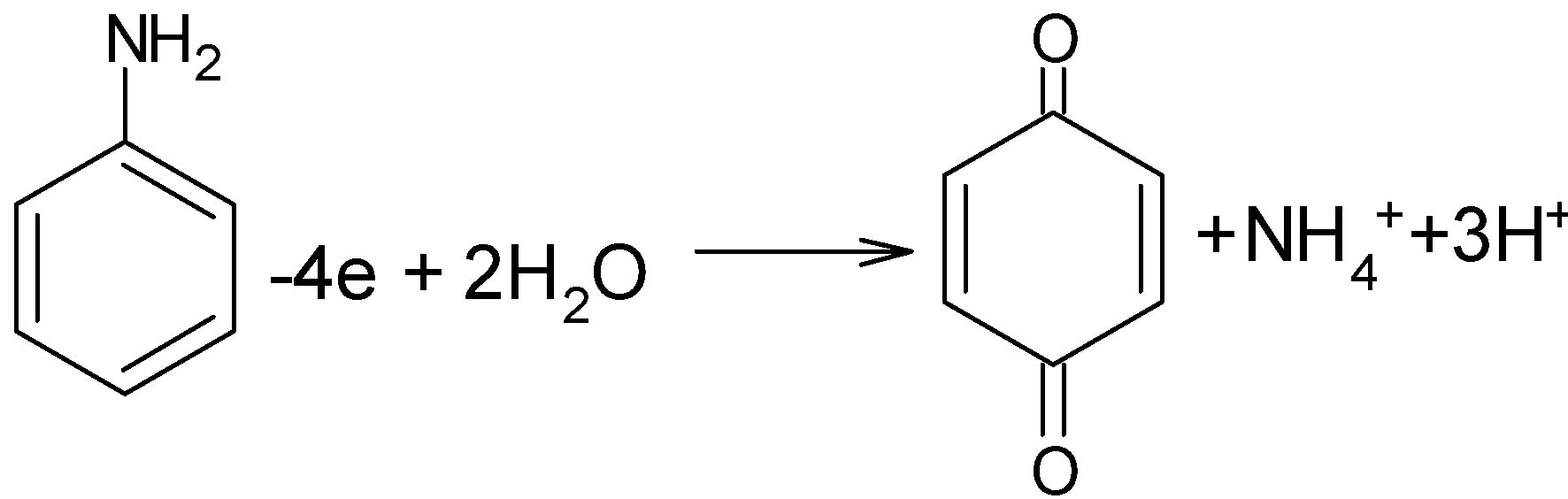
The reaction of the oxidation of the aniline is carried out through the removal of electrons from the aniline in an acidic medium. The nascent oxygen produced from the potassium dichromate in sulfuric acid reacts with the aniline to form the $\text{1,4-benzoquinone}$ with the removal of $\text{NH}_{\text{4}}^{\text{+}}$ ammonium ion and protons. The yield of the product is $82{\scriptstyle{}^{0}/{}_{0}}$.The reaction can be shown as below,
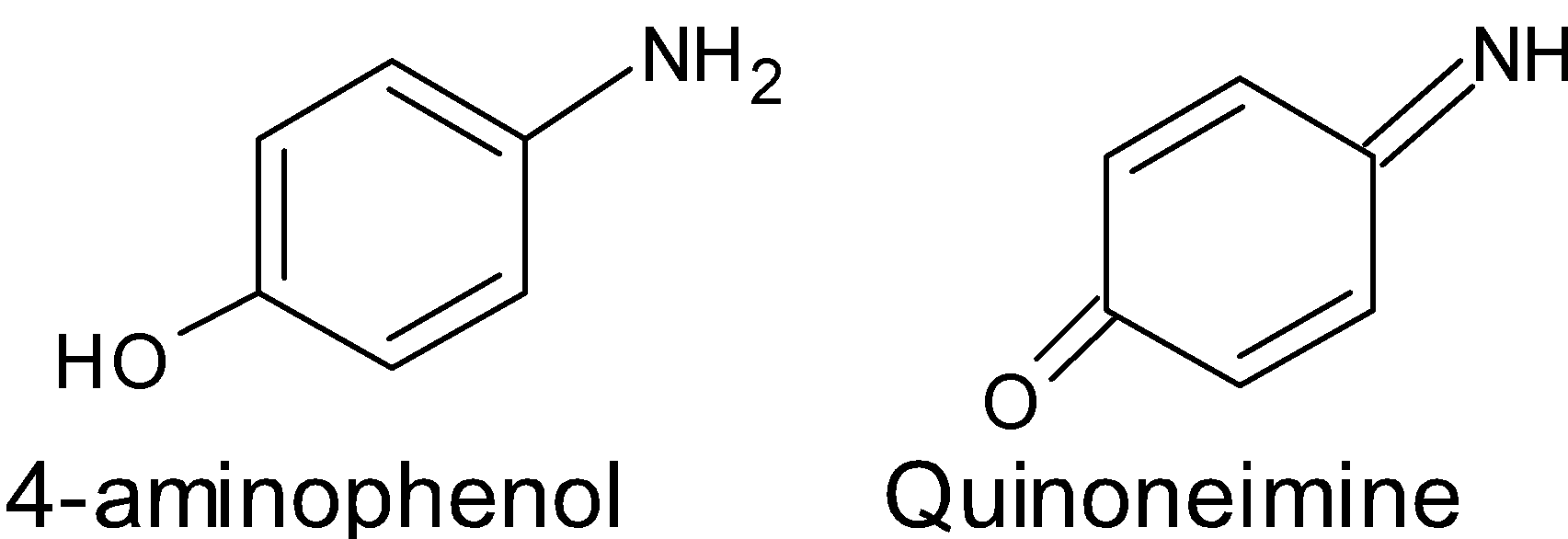
The reaction between the aniline in presence of potassium dichromate in acidic medium to give $\text{1,4-benzoquinone}$ goes through intermediates such as $\text{4-aminophenol}$and quinoneimine.The structure of the intermediates are as shown below:
Note:
On comparing this reaction with the phenols, phenols do give the benzoquinone as a product.one can say that aniline follows the similar mechanism as the phenol, though the detail mechanism for the aniline to benzoquinone in presence of acidified ${{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ is not known.
Complete answer:
Amines in which the nitrogen atom is directly bonded to the one or more aromatic rings are called the aromatic amine. Aniline is an example of primary $({{1}^{0}})$ aromatic amine.

Aromatic amines like the aniline $\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{-N}{{\text{H}}_{\text{2}}}\text{)}$ undergo various type of reactions. One such reaction is oxidation.
An oxidation reaction is defined as the addition of oxygen to an organic compound. It is also a reaction of the removal of hydrogen or removal of an electron.
Oxidation of amines gives different products depending on the nature of amine and oxidizing agent. Amines undergo oxidation by a powerful oxidizing agents such as $\text{KMn}{{\text{O}}_{\text{4}}}$ hydrogen peroxide,${{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$, etc.
In the case of aromatic amine, because of the high electron density on the benzene ring they are readily oxidized on exposure to air or oxidizing agents.
The amine undergoes the oxidation reaction in presence of acidified potassium dichromate. Potassium dichromate ${{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ is a very strong oxidizing agent that readily adds the oxygen atom in the compound. In presence of dil.${{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}$, the ${{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ gives the three atoms of oxygen as follows.
${{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\text{+4}{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\to {{\text{K}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{+Cr2(S}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}\text{+3}\left[ \text{O} \right]\text{+2}{{\text{H}}_{\text{2}}}\text{O}$
These three nascent oxygen atoms oxidized then aniline effectively.

Hence, (A) is the correct option.
Additional information:
The reaction of the oxidation of the aniline is carried out through the removal of electrons from the aniline in an acidic medium. The nascent oxygen produced from the potassium dichromate in sulfuric acid reacts with the aniline to form the $\text{1,4-benzoquinone}$ with the removal of $\text{NH}_{\text{4}}^{\text{+}}$ ammonium ion and protons. The yield of the product is $82{\scriptstyle{}^{0}/{}_{0}}$.The reaction can be shown as below,

The reaction between the aniline in presence of potassium dichromate in acidic medium to give $\text{1,4-benzoquinone}$ goes through intermediates such as $\text{4-aminophenol}$and quinoneimine.The structure of the intermediates are as shown below:

Note:
On comparing this reaction with the phenols, phenols do give the benzoquinone as a product.one can say that aniline follows the similar mechanism as the phenol, though the detail mechanism for the aniline to benzoquinone in presence of acidified ${{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ is not known.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




