
Oxidation number of sulphur in peroxomonosulphuric acid $({H}_{2}S{O}_{5})$ is :
A. +4
B. +2
C. +6
D. -2
Answer
596.1k+ views
Hint: The oxidation state or oxidation number is defined as the no. of electrons lost or gained by the atom of that element in the compound. It is used to keep track of how many electrons an atom has.
Complete step by step answer:
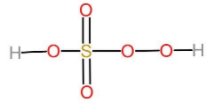
The oxidation states of sulphur are -2, -1, 0, +1, +2, +3, +4, +5, and +6. It is strongly acidic oxide. But generally, Sulphur shows only -2, 0, +2, +4 and +6 oxidation states. peroxo acids of sulphur are those acids which have a O - O bond in them. Let us talk about peroxomonosulphuric acid. It is also known as the persulphuric acid, peroxysulphuric acid, or Caro's acid. In this acid, the central sulphur adopts its tetrahedral geometry. The molecular formula of peroxomonosulphuric acid can be written as $HO-O-S({O}_{2})-OH$. It is one of the strongest oxidants known. The oxidation state of sulphur is peroxomonosulphuric acid is +6. The oxidation state of sulphur in ${H}_{2}S{O}_{5}$ is evident from the following structure. The two oxygen atoms attached to the sulphur with double bonds are known as peroxides and have the oxidation state -1. And the other two oxygen atoms attached to sulphur with single bonds have the oxidation state -2. Now to form a covalent bond with these atoms sulphur has to donate its six electrons to the oxygen atoms. And therefore, the oxidation state of sulphur is +6.
Therefore, the correct option is option (C).
Peroxomonosulphuric acid is used for treatment of swimming pools and denature cleaning. Alkali salts of peroxomonosulphuric acids are used for delignification of wood. It is also used in laboratories.
Note: Students tend to confuse between ${H}_{2}S{O}_{5}$ and ${H}_{2}{S}_{2}{O}_{5}$. Please see that these two compounds are totally different from each other. The oxidation state of sulphur in ${H}_{2}S{O}_{5}$ is +6 whereas, ${H}_{2}{S}_{2}{O}_{5}$ has two sulphur atoms and thus has two different oxidation states i.e., +3 and +5.
Complete step by step answer:
The oxidation states of sulphur are -2, -1, 0, +1, +2, +3, +4, +5, and +6. It is strongly acidic oxide. But generally, Sulphur shows only -2, 0, +2, +4 and +6 oxidation states. peroxo acids of sulphur are those acids which have a O - O bond in them. Let us talk about peroxomonosulphuric acid. It is also known as the persulphuric acid, peroxysulphuric acid, or Caro's acid. In this acid, the central sulphur adopts its tetrahedral geometry. The molecular formula of peroxomonosulphuric acid can be written as $HO-O-S({O}_{2})-OH$. It is one of the strongest oxidants known. The oxidation state of sulphur is peroxomonosulphuric acid is +6. The oxidation state of sulphur in ${H}_{2}S{O}_{5}$ is evident from the following structure. The two oxygen atoms attached to the sulphur with double bonds are known as peroxides and have the oxidation state -1. And the other two oxygen atoms attached to sulphur with single bonds have the oxidation state -2. Now to form a covalent bond with these atoms sulphur has to donate its six electrons to the oxygen atoms. And therefore, the oxidation state of sulphur is +6.

Therefore, the correct option is option (C).
Peroxomonosulphuric acid is used for treatment of swimming pools and denature cleaning. Alkali salts of peroxomonosulphuric acids are used for delignification of wood. It is also used in laboratories.
Note: Students tend to confuse between ${H}_{2}S{O}_{5}$ and ${H}_{2}{S}_{2}{O}_{5}$. Please see that these two compounds are totally different from each other. The oxidation state of sulphur in ${H}_{2}S{O}_{5}$ is +6 whereas, ${H}_{2}{S}_{2}{O}_{5}$ has two sulphur atoms and thus has two different oxidation states i.e., +3 and +5.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




