
Oxidation number of carbon in $SC{{N}^{-}}$ ion is:
Answer
518.4k+ views
Hint: To find the oxidation number of the given element in the compound or ion we need to know the oxidation numbers of other elements so, the oxidation number of sulfur will be -2 and the oxidation number of nitrogen will be -3. Since it is a single negative ion, the sum of oxidation number of sulfur, carbon, and nitrogen will be equal to -1.
Complete answer:
The oxidation number, also known as the oxidation number, is a state of an element that is used to describe the property of the composition of a compound with other elements.
To find the oxidation number of the given element in the compound or ion we need to know the oxidation numbers of other elements so, the oxidation number of sulfur will be -2 and the oxidation number of nitrogen will be -3. Since it is a single negative ion, the sum of oxidation number of sulfur, carbon, and nitrogen will be equal to -1.
Let us take the oxidation number of carbon as x. Now putting the values, we get:
$-2+x-3=-1$
$x-5=-1$
$x=+5-1$
$x=+4$
So, the oxidation number of carbon in $SC{{N}^{-}}$ will be +4.
The structure of $SC{{N}^{-}}$ ion is given below:
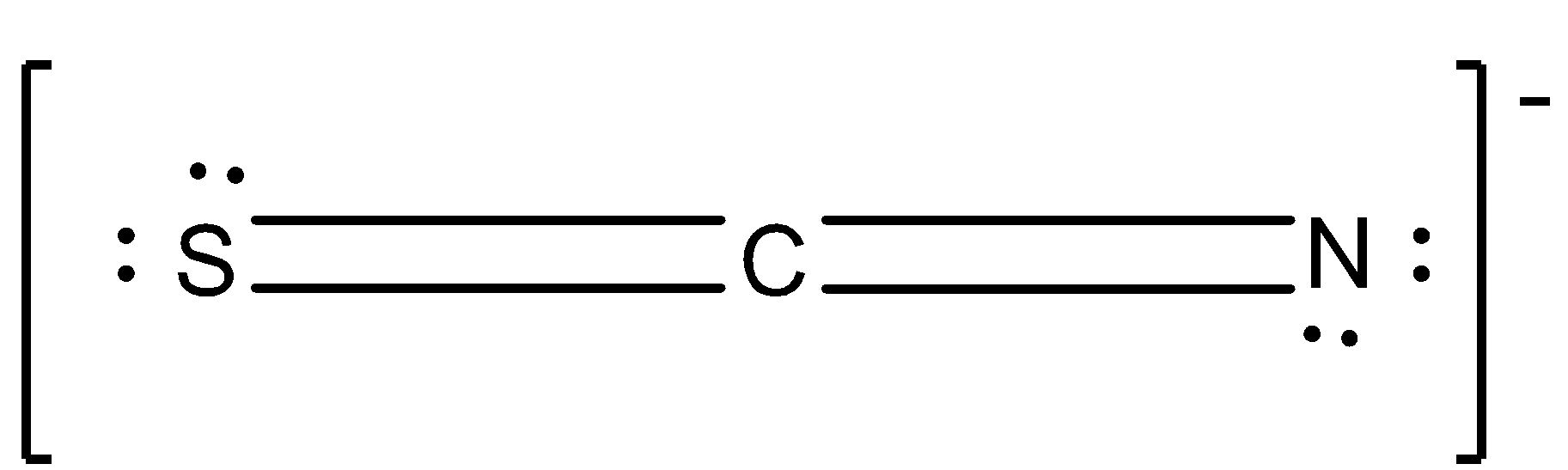
There can be many equivalent structures of this compound because of resonance in the compound.
Note:
With the help of finding the number of groups of the element then we can easily find the oxidation number of the element like if the group is 16 then the oxidation number will be -2, i.e., oxygen and sulfur.
Complete answer:
The oxidation number, also known as the oxidation number, is a state of an element that is used to describe the property of the composition of a compound with other elements.
To find the oxidation number of the given element in the compound or ion we need to know the oxidation numbers of other elements so, the oxidation number of sulfur will be -2 and the oxidation number of nitrogen will be -3. Since it is a single negative ion, the sum of oxidation number of sulfur, carbon, and nitrogen will be equal to -1.
Let us take the oxidation number of carbon as x. Now putting the values, we get:
$-2+x-3=-1$
$x-5=-1$
$x=+5-1$
$x=+4$
So, the oxidation number of carbon in $SC{{N}^{-}}$ will be +4.
The structure of $SC{{N}^{-}}$ ion is given below:

There can be many equivalent structures of this compound because of resonance in the compound.
Note:
With the help of finding the number of groups of the element then we can easily find the oxidation number of the element like if the group is 16 then the oxidation number will be -2, i.e., oxygen and sulfur.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




