
Out of the following isomeric alcohols containing five carbon atoms, the alcohol that exhibits optical isomerism is:
A. \[1 - \]pentanol
B. \[2 - \]pentanol
C. \[3 - \]pentanol
D. \[2 - \]methyl\[ - 2 - \]butanol
E. \[2,2 - \]dimethyl\[ - 1 - \]propanol
Answer
561k+ views
Hint: Optical isomerism is a property of a compound by which it changes the polarity of a plane polarized light. The primary condition for optical isomerism is the molecule should be chiral.
Complete step by step answer:
Optical isomers are a set of two compounds which contains the same atoms and same bonds but differ in the spatial arrangements of the bonded atoms. This results in the two compounds being non-superimposable mirror images of each other.
The non-superimposable mirror images are labeled as enantiomers. The compounds or molecules which follow the above conditions of non superimposable mirror image are called as chiral compounds or molecules.
When a plane polarized light is passed through a solution containing chiral compounds the rotation of the light is affected. The compounds which show such property are also termed as stereoisomers. The two isomers have an equal degree of rotation one in clockwise and another in anticlockwise direction. Thus one is positive optical rotation and the other is negative optical rotation.
A molecule must contain a chiral centre or chiral bond or a chiral plane to show stereoisomerism. A chiral centre is the centre which is attached to four different substituents. Let us find which of the given set of alcohols have a chiral centre. This is identified by the molecular structure of the compound.
A. \[1 - \]pentanol.
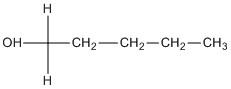
The compound does not contain any chiral centre.
B. \[2 - \]pentanol
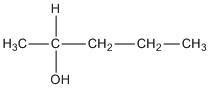
The compound contains a chiral centre as the carbon is attached to four different substituents.
C. \[3 - \]pentanol
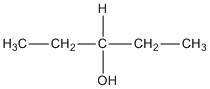
The compound does not contain a chiral centre.
D. \[2 - \]methyl\[ - 2 - \]butanol
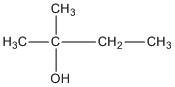
The molecule does not contain a chiral centre.
E. \[2,2 - \]dimethyl\[ - 1 - \]propanol
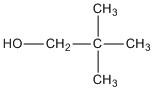
The molecule does not contain a chiral centre.
Thus out of the following isomeric alcohols containing five carbon atoms, the alcohol that exhibits optical isomerism is \[2 - \]pentanol as it contains a chiral centre, i.e. option B is the correct answer.
Note:
The difference between chiral and achiral molecules is determined by identifying the plane of symmetry in the molecule. If all the substituents attached to a carbon atom are different then no plane of symmetry exists and the molecule is a chiral molecule.
Complete step by step answer:
Optical isomers are a set of two compounds which contains the same atoms and same bonds but differ in the spatial arrangements of the bonded atoms. This results in the two compounds being non-superimposable mirror images of each other.
The non-superimposable mirror images are labeled as enantiomers. The compounds or molecules which follow the above conditions of non superimposable mirror image are called as chiral compounds or molecules.
When a plane polarized light is passed through a solution containing chiral compounds the rotation of the light is affected. The compounds which show such property are also termed as stereoisomers. The two isomers have an equal degree of rotation one in clockwise and another in anticlockwise direction. Thus one is positive optical rotation and the other is negative optical rotation.
A molecule must contain a chiral centre or chiral bond or a chiral plane to show stereoisomerism. A chiral centre is the centre which is attached to four different substituents. Let us find which of the given set of alcohols have a chiral centre. This is identified by the molecular structure of the compound.
A. \[1 - \]pentanol.

The compound does not contain any chiral centre.
B. \[2 - \]pentanol

The compound contains a chiral centre as the carbon is attached to four different substituents.
C. \[3 - \]pentanol

The compound does not contain a chiral centre.
D. \[2 - \]methyl\[ - 2 - \]butanol

The molecule does not contain a chiral centre.
E. \[2,2 - \]dimethyl\[ - 1 - \]propanol

The molecule does not contain a chiral centre.
Thus out of the following isomeric alcohols containing five carbon atoms, the alcohol that exhibits optical isomerism is \[2 - \]pentanol as it contains a chiral centre, i.e. option B is the correct answer.
Note:
The difference between chiral and achiral molecules is determined by identifying the plane of symmetry in the molecule. If all the substituents attached to a carbon atom are different then no plane of symmetry exists and the molecule is a chiral molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




