
Osmoscope is used for
(a)Measuring TP (turgor pressure)
(b)Measuring OP (osmotic pressure)
(c)Measuring W (water potential)
(d)Demonstrating osmosis
Answer
600.6k+ views
Hint: It is a special type of process of movement of solvent from an area of a higher number to an area of lower number through a semipermeable membrane until the number of molecules on both sides is equal.
Complete answer:
Osmoscope is an instrument used for the measurement of osmosis. The instrument contains a beaker into which a semipermeable membrane. The inner of the semipermeable membrane contains a solution in which the solute (that is dissolved in the liquid or solvent) is more in number than the solvent. At the outer sides of the semipermeable membrane, the only solvent is placed to witness the process of movement of solvent where it is more in number to the solution, where the solvent is less in number.
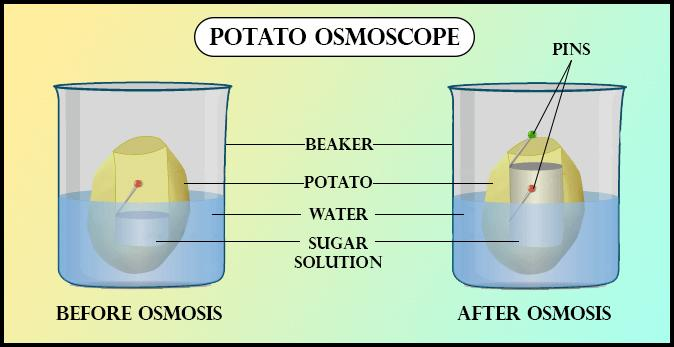
Example of potato osmoscope: Peel off the skin of a large-sized potato with the help of a scalpel. Cut its one end to make its base flat. Make a hollow cavity in the potato almost up to the bottom, Put the sugar solution into the cavity and mark the level by inserting a pin in the wall of the cavity of the potato. Place the potato in a beaker containing water. After sometime, it will be noticed that the level in the cavity rises. It is due to the phenomenon of osmosis. The experiments demonstrate that living cells of potato act as a differentially permeable membrane.
So, the correct answer is, ‘Demonstrating osmosis.’
Note: The other definition of osmosis can be the migration of solvent from a hypotonic solution (having a lower number of molecules of solute) to the hypertonic solution (having a higher number of molecules of solute) through a semipermeable membrane to keep the number of molecules on both sides equal.
Complete answer:
Osmoscope is an instrument used for the measurement of osmosis. The instrument contains a beaker into which a semipermeable membrane. The inner of the semipermeable membrane contains a solution in which the solute (that is dissolved in the liquid or solvent) is more in number than the solvent. At the outer sides of the semipermeable membrane, the only solvent is placed to witness the process of movement of solvent where it is more in number to the solution, where the solvent is less in number.
Example of potato osmoscope: Peel off the skin of a large-sized potato with the help of a scalpel. Cut its one end to make its base flat. Make a hollow cavity in the potato almost up to the bottom, Put the sugar solution into the cavity and mark the level by inserting a pin in the wall of the cavity of the potato. Place the potato in a beaker containing water. After sometime, it will be noticed that the level in the cavity rises. It is due to the phenomenon of osmosis. The experiments demonstrate that living cells of potato act as a differentially permeable membrane.
So, the correct answer is, ‘Demonstrating osmosis.’

Note: The other definition of osmosis can be the migration of solvent from a hypotonic solution (having a lower number of molecules of solute) to the hypertonic solution (having a higher number of molecules of solute) through a semipermeable membrane to keep the number of molecules on both sides equal.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




