
Orlon has monomeric unit of :
(A) acrolein
(B) glycol
(C) vinyl cyanide
(D) isoprene
Answer
592.2k+ views
Hint: Orlon is the commercial name for the polymer polyacrylonitrile. Since orlon is a homo polymer, it is easy to find the repeating unit. The repeating unit found is the monomer for orlon also called as polyacrylonitrile.
Complete answer:
-Orlon is a synthetic acrylic fiber, which is used in the textile industry. The fiber is resistant to sunlight and atmospheric gases.
-This property of Orlon makes it suitable for outdoor uses. It is resistant to shrinkage and has a soft and warm feel. It is used in the manufacture of overcoating. Dress fabrics. Knitted wear, and washable woven sportswear.
-Orlon is an addition polymer. Let us draw the structure of Orlon to find the repeating unit.
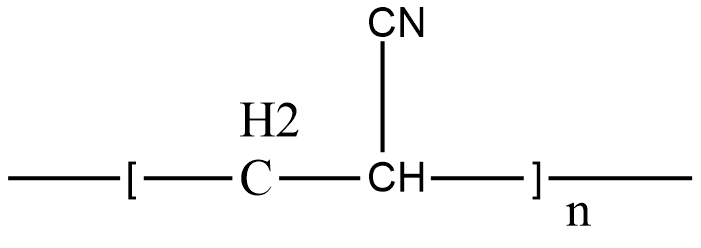
From the above structure we find that the repeating structure is acrylonitrile.
The common name for acrylonitrile is vinyl cyanide. This is because the common name for the nitrile functional group is cyanide and the nitrile functional group is attached at the vinyl position of the C=C double bond, hence the name vinyl cyanide.
The structure for vinyl cyanide is given below:
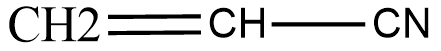
So, the correct answer is “Option C”.
Note: In the options given, acrolein is one of the options. Do not get confused between acrolein and acrylonitrile as acrolein has an aldehyde functional group and acryl nitrile has a nitrile functional group.
Complete answer:
-Orlon is a synthetic acrylic fiber, which is used in the textile industry. The fiber is resistant to sunlight and atmospheric gases.
-This property of Orlon makes it suitable for outdoor uses. It is resistant to shrinkage and has a soft and warm feel. It is used in the manufacture of overcoating. Dress fabrics. Knitted wear, and washable woven sportswear.
-Orlon is an addition polymer. Let us draw the structure of Orlon to find the repeating unit.

From the above structure we find that the repeating structure is acrylonitrile.
The common name for acrylonitrile is vinyl cyanide. This is because the common name for the nitrile functional group is cyanide and the nitrile functional group is attached at the vinyl position of the C=C double bond, hence the name vinyl cyanide.
The structure for vinyl cyanide is given below:
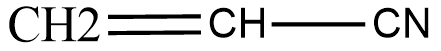
So, the correct answer is “Option C”.
Note: In the options given, acrolein is one of the options. Do not get confused between acrolein and acrylonitrile as acrolein has an aldehyde functional group and acryl nitrile has a nitrile functional group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




