
On hydrolysis of sodium carbonate, the reaction takes place between?
a.) \[N{{a}^{+}}\] and water
b.) \[N{{a}^{+}}\] and \[O{{H}^{-}}\]
c.) \[CO_{3}^{2-}\] and water
d.) \[CO_{3}^{2-}\] and \[{{H}^{+}}\]
Answer
601.8k+ views
Hint: Hydrolysis, as the name suggests, is a reaction which includes the breaking up of compounds with the help of water. Water generally acts as a nucleophile in such reactions.
Complete step by step answer:
Hydrolysis is a reaction which includes breaking down a compound due to reaction with water.
The chemical name of sodium bicarbonate is written as – \[N{{a}_{2}}C{{O}_{3}}\].
This compound is a salt. As we can see, this compound is made up of 2 sodium cations and one carbonate anion, which bond together due to electrostatic force of attraction between them. It is alkaline in nature.
On reaction with one mole of water, it gives sodium hydroxide and sodium bicarbonate.
\[N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O\rightleftharpoons NaOH+NaHC{{O}_{3}}\]
If we write it as individual ions, the reaction become –
\[2N{{a}^{+}}+CO_{3}^{2-}+{{H}_{2}}O\rightleftharpoons N{{a}^{+}}+O{{H}^{-}}+N{{a}^{+}}+HCO_{3}^{-}\]
On cancelling the common terms, we get –
\[CO_{3}^{2-}+{{H}_{2}}O\rightleftharpoons O{{H}^{-}}+HCO_{3}^{-}\]
Therefore, we can see that sodium finishes in this reaction. Hence, we can say that the reaction takes place between carbonate ion and water.
Therefore, the answer is – option (c) .
Additional Information:
Sodium carbonate is an ionic compound. Its structure is given as –
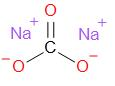 Note: Sodium bicarbonate has many common names such as - washing soda, soda ash and soda crystals. It occurs as a white coloured water solid salt. It has a strong alkaline taste. It is very widely used in detergents, as a water softener, paper industry, glass manufacture industry, etc. Not only this, it is also used in the food industry as an acidity regulator.
Note: Sodium bicarbonate has many common names such as - washing soda, soda ash and soda crystals. It occurs as a white coloured water solid salt. It has a strong alkaline taste. It is very widely used in detergents, as a water softener, paper industry, glass manufacture industry, etc. Not only this, it is also used in the food industry as an acidity regulator.
Complete step by step answer:
Hydrolysis is a reaction which includes breaking down a compound due to reaction with water.
The chemical name of sodium bicarbonate is written as – \[N{{a}_{2}}C{{O}_{3}}\].
This compound is a salt. As we can see, this compound is made up of 2 sodium cations and one carbonate anion, which bond together due to electrostatic force of attraction between them. It is alkaline in nature.
On reaction with one mole of water, it gives sodium hydroxide and sodium bicarbonate.
\[N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O\rightleftharpoons NaOH+NaHC{{O}_{3}}\]
If we write it as individual ions, the reaction become –
\[2N{{a}^{+}}+CO_{3}^{2-}+{{H}_{2}}O\rightleftharpoons N{{a}^{+}}+O{{H}^{-}}+N{{a}^{+}}+HCO_{3}^{-}\]
On cancelling the common terms, we get –
\[CO_{3}^{2-}+{{H}_{2}}O\rightleftharpoons O{{H}^{-}}+HCO_{3}^{-}\]
Therefore, we can see that sodium finishes in this reaction. Hence, we can say that the reaction takes place between carbonate ion and water.
Therefore, the answer is – option (c) .
Additional Information:
Sodium carbonate is an ionic compound. Its structure is given as –

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




