
what is the octet rule? How do you appreciate the role of the octet rule in explaining the chemical properties of elements?
Answer
573.6k+ views
Hint: Octet rule helps in deciding the stability of an element and its tendency to either give up electrons or gain electrons. It is also an important rule that dictates the electronic configuration of an element.
Complete step by step answer:
Octet rule can be stated as the requirement of elements to possess eight electrons in their valence shell to gain stability.As mentioned above, in order for an element to fulfill the octet rule it must either lose electrons or gain electrons. After doing so, these elements resemble the characteristics of a noble gas.This phenomenon where in the element that has octet configuration has particular chemical characteristics which can be important in classifying and characterizing the elements that have not yet been discovered or for elements that have yet to be added into the periodic table. This rule is also responsible for the formation of all bonds as the bond formation occurs between two elements that do not have octet configuration. For example, in covalent bonds a pair of electrons are shared. In an ionic bond, an electron is transferred from one element to another. This is also important in formation of anions and cations. As an anion is formed by an element by accepting electrons and a cation is formed by an element by donating or releasing electrons. The ionic bond as mentioned before, can be demonstrated in the diagram below:
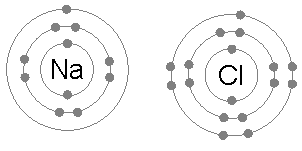
Here, the lone electron present in the outermost shell of sodium gets transferred to chlorine.
In the end, what we need to remember is that all elements strive to attain the octet configuration. Once it attains that it becomes highly stable and assumes the characteristics of a noble gas.
It can also dictate the rules of electronegativity and electropositivity of an element.
Note: The octet rule is used to provide stability to an atom. An element that finally attains the octet configuration will become inert and chemically unreactive. The octet rule also dictates the formation of bonds between two chemical species.
Complete step by step answer:
Octet rule can be stated as the requirement of elements to possess eight electrons in their valence shell to gain stability.As mentioned above, in order for an element to fulfill the octet rule it must either lose electrons or gain electrons. After doing so, these elements resemble the characteristics of a noble gas.This phenomenon where in the element that has octet configuration has particular chemical characteristics which can be important in classifying and characterizing the elements that have not yet been discovered or for elements that have yet to be added into the periodic table. This rule is also responsible for the formation of all bonds as the bond formation occurs between two elements that do not have octet configuration. For example, in covalent bonds a pair of electrons are shared. In an ionic bond, an electron is transferred from one element to another. This is also important in formation of anions and cations. As an anion is formed by an element by accepting electrons and a cation is formed by an element by donating or releasing electrons. The ionic bond as mentioned before, can be demonstrated in the diagram below:

Here, the lone electron present in the outermost shell of sodium gets transferred to chlorine.
In the end, what we need to remember is that all elements strive to attain the octet configuration. Once it attains that it becomes highly stable and assumes the characteristics of a noble gas.
It can also dictate the rules of electronegativity and electropositivity of an element.
Note: The octet rule is used to provide stability to an atom. An element that finally attains the octet configuration will become inert and chemically unreactive. The octet rule also dictates the formation of bonds between two chemical species.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




