
Number of valence electron in a carbon atom is
A. 3
B. 1
C. 4
D. None
Answer
565.5k+ views
Hint:for this type of question to find the valence electron of a carbon atom firstly we have to do the electronic configuration of the carbon atom, after finding the electronic configuration we have to arrange electrons in electronic configuration now to calculate number of valence electrons we have to find out the outermost shell and count the number of electrons in outermost shell. And these are called valence electrons.
Complete step-by-step answer:$\therefore $we all know that atomic number of the carbon atom is 6.
So, there will be 6 electrons in the carbon atom and 6 protons in the carbon atom to make atom neutral.
So, the atomic mass of the carbon atom will be 12 gram.
Now we will make an electronic configuration of carbon atoms.
$C(6) = 1{s^2},2{s^2},2{p^2}$
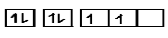
$1s\,\,\,\,\,\,\,2s\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,2p$
Now we have seen in the electronic configuration of carbon atom that we have used only 2 shells
So, the outermost shell be 2
$\therefore $number of electrons in the shell number 2 is 4 (two electrons are from 2s and two electrons are from 2p)
So after adding both orbitals of electrons we get a total number of electrons in the outermost shell is 4.
$\therefore $an atom can share only its outermost electrons of the shell. Hence, carbon can share its only 4 electrons to the other atom.
Therefore, valency of the carbon atom is 4.it can share its only 4 electrons from an atom of carbon
So, the number of valence electrons in the carbon atom will be 4.
Therefore, the correct answer will be option number C.
Note:If we find the number of electrons in an outermost shell of an atom then we can say I have counted the valence electron of that atom means valence electrons are the electrons that are found in the outermost shell of an atom, valence electrons are the electrons that are used to share by an atom to form the Sigma bonds and Pi bonds i.e. to make the covalent bonds.
Complete step-by-step answer:$\therefore $we all know that atomic number of the carbon atom is 6.
So, there will be 6 electrons in the carbon atom and 6 protons in the carbon atom to make atom neutral.
So, the atomic mass of the carbon atom will be 12 gram.
Now we will make an electronic configuration of carbon atoms.
$C(6) = 1{s^2},2{s^2},2{p^2}$

$1s\,\,\,\,\,\,\,2s\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,2p$
Now we have seen in the electronic configuration of carbon atom that we have used only 2 shells
So, the outermost shell be 2
$\therefore $number of electrons in the shell number 2 is 4 (two electrons are from 2s and two electrons are from 2p)
So after adding both orbitals of electrons we get a total number of electrons in the outermost shell is 4.
$\therefore $an atom can share only its outermost electrons of the shell. Hence, carbon can share its only 4 electrons to the other atom.
Therefore, valency of the carbon atom is 4.it can share its only 4 electrons from an atom of carbon
So, the number of valence electrons in the carbon atom will be 4.
Therefore, the correct answer will be option number C.
Note:If we find the number of electrons in an outermost shell of an atom then we can say I have counted the valence electron of that atom means valence electrons are the electrons that are found in the outermost shell of an atom, valence electrons are the electrons that are used to share by an atom to form the Sigma bonds and Pi bonds i.e. to make the covalent bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




