
Number of ionisable and non ionisable $C{l^ - }$ ions in $CoC{l_3}.5N{H_3}$ respectively are, if one mole of it reacts with two mole of silver nitrate completely and forms ppt.
A.$3,0$
B.$2,1$
C.$1,2$
D.$0,3$
Answer
569.4k+ views
Hint: $CoC{l_3}.5N{H_3}$ is a coordination complex. This complex reacts with silver nitrate and precipitates. The number of ionizable and non ionizable ions depends on the type of valency in a complex.
Complete step by step answer:
-The IUPAC name of $CoC{l_3}.5N{H_3}$ is pentaamminechlorocobalt $\left( {III} \right)$ chloride.
-The structure of $CoC{l_3}.5N{H_3}$ is given below as follows:
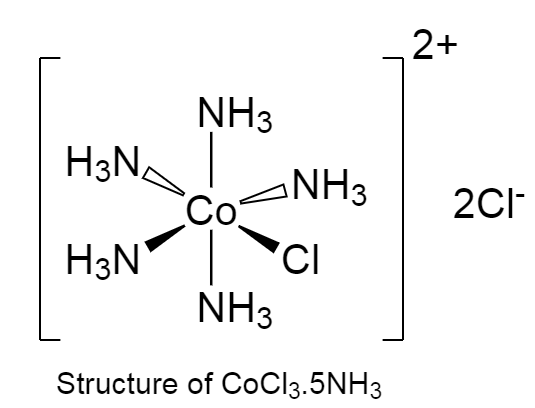
-In the given coordination complex, five $N{H_3}$ molecule are directly attached to cobalt metal.
-Only one chloride ion is attached to cobalt metal.
-The rest of the chloride ion is satisfying the oxidation state of the metal.
-The chloride ion that is satisfying the oxidation of metal is called primary valency.
-And the one that is attached directly to metal is called secondary valency.
-Primary valency consists of ionisable ions whereas secondary valency consists of non ionizable ions.
-Primary valencies are satisfied by the negative charges whereas secondary valencies are satisfied by the positive charge or neutral species.
-The number of secondary valence shown by the metal is fixed.
-Thus, therefore the formula for the complex is $\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]C{l_2}$ .
-One mole of $CoC{l_3}.5N{H_3}$ reacted with two moles of silver nitrate to form precipitate.
-Since the primary valency of chloride ions in the complex is two, therefore the number of ionisable ions will also be.
-Similarly for non ionizable ions, it is the same as secondary valency that is one.
-Thus, the number of ionizable ions is two and non ionisable ions is one.
So, the correct answer is option B) $2,1$ .
Note:Primary valency is the number of ions that satisfies the charge of the ion. Secondary valency is the same as the coordination number of a complex. In the structure of the complex, the primary valency is represented by a full line whereas the secondary valency is represented by a dotted line.
Complete step by step answer:
-The IUPAC name of $CoC{l_3}.5N{H_3}$ is pentaamminechlorocobalt $\left( {III} \right)$ chloride.
-The structure of $CoC{l_3}.5N{H_3}$ is given below as follows:

-In the given coordination complex, five $N{H_3}$ molecule are directly attached to cobalt metal.
-Only one chloride ion is attached to cobalt metal.
-The rest of the chloride ion is satisfying the oxidation state of the metal.
-The chloride ion that is satisfying the oxidation of metal is called primary valency.
-And the one that is attached directly to metal is called secondary valency.
-Primary valency consists of ionisable ions whereas secondary valency consists of non ionizable ions.
-Primary valencies are satisfied by the negative charges whereas secondary valencies are satisfied by the positive charge or neutral species.
-The number of secondary valence shown by the metal is fixed.
-Thus, therefore the formula for the complex is $\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]C{l_2}$ .
-One mole of $CoC{l_3}.5N{H_3}$ reacted with two moles of silver nitrate to form precipitate.
-Since the primary valency of chloride ions in the complex is two, therefore the number of ionisable ions will also be.
-Similarly for non ionizable ions, it is the same as secondary valency that is one.
-Thus, the number of ionizable ions is two and non ionisable ions is one.
So, the correct answer is option B) $2,1$ .
Note:Primary valency is the number of ions that satisfies the charge of the ion. Secondary valency is the same as the coordination number of a complex. In the structure of the complex, the primary valency is represented by a full line whereas the secondary valency is represented by a dotted line.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




