
Number of covalent bonds present in $Ca{(OCl)_2}$ .
Answer
561k+ views
Hint: For solving, this question we need to first understand the meaning of covalent bonds. We can define a covalent bond as a type of a molecular bond in which a chemical bond is formed by sharing of the electron pairs between different atoms.
Complete step by step answer:
As we know that, the electron pairs shared between atoms are known as bonding pairs or shared pairs. They possess a stable balance of attractive and repulsive forces between the atoms, when they share electrons. Such type of bonding between atoms is known as covalent bonding.
Here, we know that a covalent bond is formed by the interatomic linkage that is formed by the sharing of an electron pair between two atoms. The binding arises from the electrostatic attraction of their nuclei for the same number of electrons.
We know that we can write $Ca{(OCl)_2}$ as $Ca(OCl)Cl$ . On the basis of this we know that there are $C{a^{ - 2}}$ ion, an $OC{l^ - }$ ion and an $C{l^ - }$ ion.
So, from the structure we can see there is a covalent bond shared between O and Cl in the atom of $OC{l^ - }$ .
To check the number of covalent bonds we will draw the structure of $OC{l^ - }$.
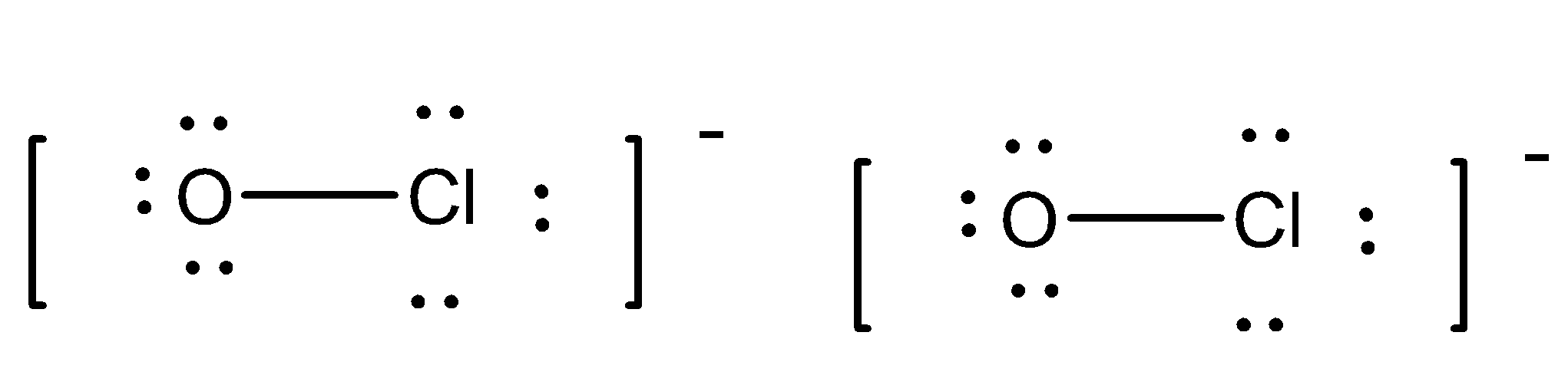
So, from the above structure diagram we can note that the given oxychloride compound has 2 covalent bonds in the molecule.
Note:
Here, an ionic bond is also shared between $C{a^{2 + }}$ , $C{l^ - }$ and $OC{l^ - }$. The transfer of electrons is referred to an ionic bond. These bonds are electrostatic in nature. It is formed by the attraction of positive and negative ions that lead to transfer of electrons. And charge separation in ionic bonds is more in comparison to covalent bonds.
Complete step by step answer:
As we know that, the electron pairs shared between atoms are known as bonding pairs or shared pairs. They possess a stable balance of attractive and repulsive forces between the atoms, when they share electrons. Such type of bonding between atoms is known as covalent bonding.
Here, we know that a covalent bond is formed by the interatomic linkage that is formed by the sharing of an electron pair between two atoms. The binding arises from the electrostatic attraction of their nuclei for the same number of electrons.
We know that we can write $Ca{(OCl)_2}$ as $Ca(OCl)Cl$ . On the basis of this we know that there are $C{a^{ - 2}}$ ion, an $OC{l^ - }$ ion and an $C{l^ - }$ ion.
So, from the structure we can see there is a covalent bond shared between O and Cl in the atom of $OC{l^ - }$ .
To check the number of covalent bonds we will draw the structure of $OC{l^ - }$.
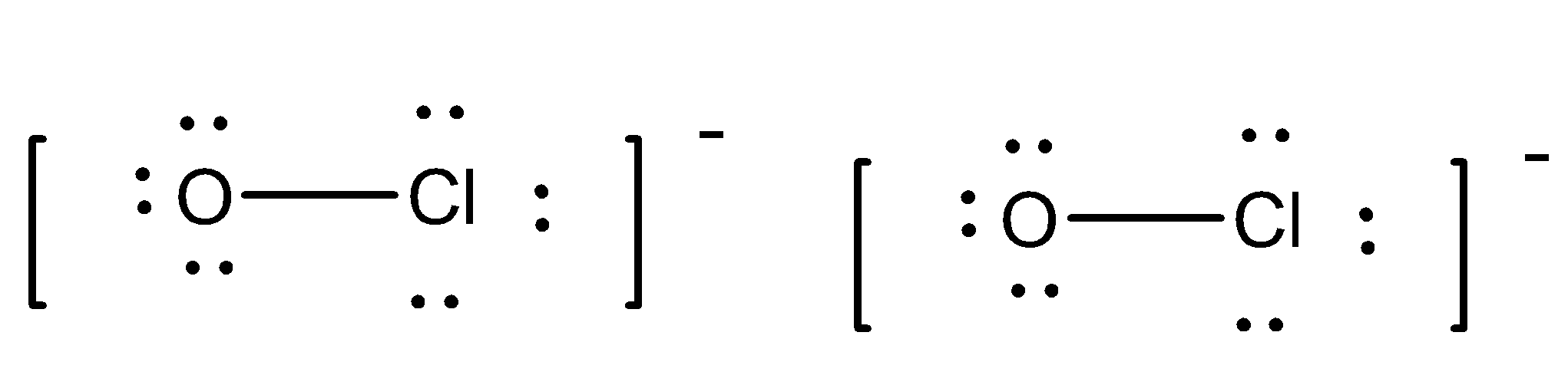
So, from the above structure diagram we can note that the given oxychloride compound has 2 covalent bonds in the molecule.
Note:
Here, an ionic bond is also shared between $C{a^{2 + }}$ , $C{l^ - }$ and $OC{l^ - }$. The transfer of electrons is referred to an ionic bond. These bonds are electrostatic in nature. It is formed by the attraction of positive and negative ions that lead to transfer of electrons. And charge separation in ionic bonds is more in comparison to covalent bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




