
N-Ethyl formamide on dehydration with $ POC{{l}_{3}}$ in presence of pyridine gives:
A.Ethyl amine
B. Ethyl cyanide
C. Ethyl isocyanide
D. Methyl isocyanide
Answer
568.2k+ views
Hint The concept of dehydration is to be used to solve this question. Double bond of the oxygen in the aldehydic group breaks and protonates the oxygen, and a complex is formed which gives out HCl and chloride ions to form the desired product.
Complete step by step solution:
In order to answer the question, we need to learn about the dehydration reactions in organic chemistry. Dehydration can be also referred to as the $ {{E}_{2}}$ elimination, that is carried out in non acidic conditions, in the presence of pyridine and phosphorous oxychloride. This procedure can be also used for dehydration of hindered $ {{2}^{0}}$ alcohols, but in case of primary alcohols, the $ S{{N}_{2}}$ mechanism takes place. Now, when $ {{3}^{0}}$ bulky alcoholic groups are dehydrated, a small amount of non Saytzeff product is formed and the reason is due to the steric hindrance. In case of $ {{2}^{0}}$ alcoholic group, dehydration with $ POC{{l}_{3}}$ takes place well as the bimolecular substitution is retarded by the stearic hindrance. Now, let us see the mechanism:
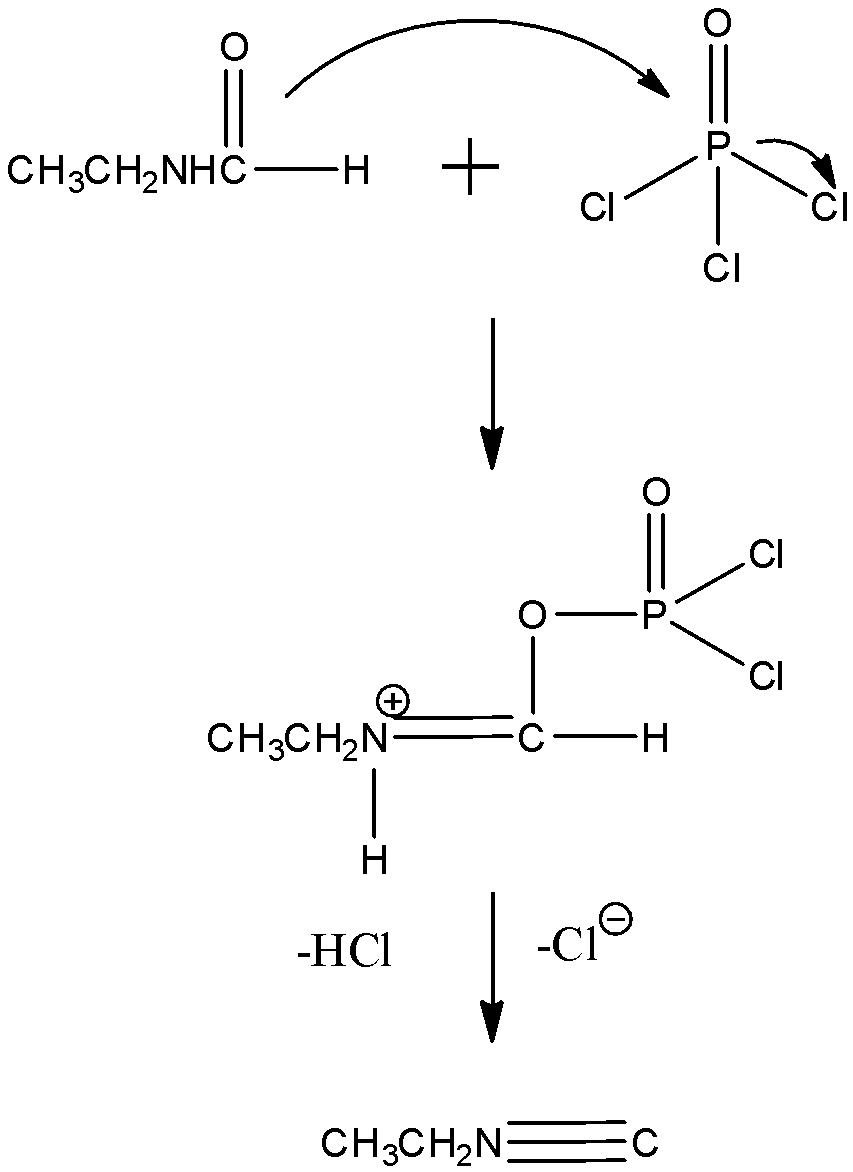
We can see that the double bond breaks and protonation takes place in the first step. Now, there is a vacancy of electrons in the oxygen atom so it gets attached with the P atom of the $ POC{{l}_{3}}$ . After getting attached it forms a complex which is very unstable. To stabilize it, dehydration takes place where HCl and the extra chloride ion is removed. Finally, we get the product as Ethyl isocyanide.
Hence, obtain the correct answer for this question as option C.
NOTE: It is to be noted that dehydration with $ POC{{l}_{3}}$ occurs more rapidly, when dehydrated by $ {{H}_{2}}S{{O}_{4}}$ , as in the first case, there is no formation of carbocation. It also involves engagement of better leaving groups and the $ {{E}_{2}}$ mechanism where the proton is captured by the pyridine base.
Complete step by step solution:
In order to answer the question, we need to learn about the dehydration reactions in organic chemistry. Dehydration can be also referred to as the $ {{E}_{2}}$ elimination, that is carried out in non acidic conditions, in the presence of pyridine and phosphorous oxychloride. This procedure can be also used for dehydration of hindered $ {{2}^{0}}$ alcohols, but in case of primary alcohols, the $ S{{N}_{2}}$ mechanism takes place. Now, when $ {{3}^{0}}$ bulky alcoholic groups are dehydrated, a small amount of non Saytzeff product is formed and the reason is due to the steric hindrance. In case of $ {{2}^{0}}$ alcoholic group, dehydration with $ POC{{l}_{3}}$ takes place well as the bimolecular substitution is retarded by the stearic hindrance. Now, let us see the mechanism:

We can see that the double bond breaks and protonation takes place in the first step. Now, there is a vacancy of electrons in the oxygen atom so it gets attached with the P atom of the $ POC{{l}_{3}}$ . After getting attached it forms a complex which is very unstable. To stabilize it, dehydration takes place where HCl and the extra chloride ion is removed. Finally, we get the product as Ethyl isocyanide.
Hence, obtain the correct answer for this question as option C.
NOTE: It is to be noted that dehydration with $ POC{{l}_{3}}$ occurs more rapidly, when dehydrated by $ {{H}_{2}}S{{O}_{4}}$ , as in the first case, there is no formation of carbocation. It also involves engagement of better leaving groups and the $ {{E}_{2}}$ mechanism where the proton is captured by the pyridine base.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




