
Natural rubber is a polymer of:
(A) 1,1-dimethylbutadiene
(B) 2-methyl-1,3-butadiene
(C) 2-chlorobuta-1,3-diene
(D) 2-chloro-but-2-ene
Answer
585k+ views
Hint: The monomer is also called Isoprene. The monomer we use is a type of a conjugated diene. The monomer will not give a positive Beilstein test in organic qualitative analysis.
Complete step by step solution:
Natural rubber has the structure as shown below.
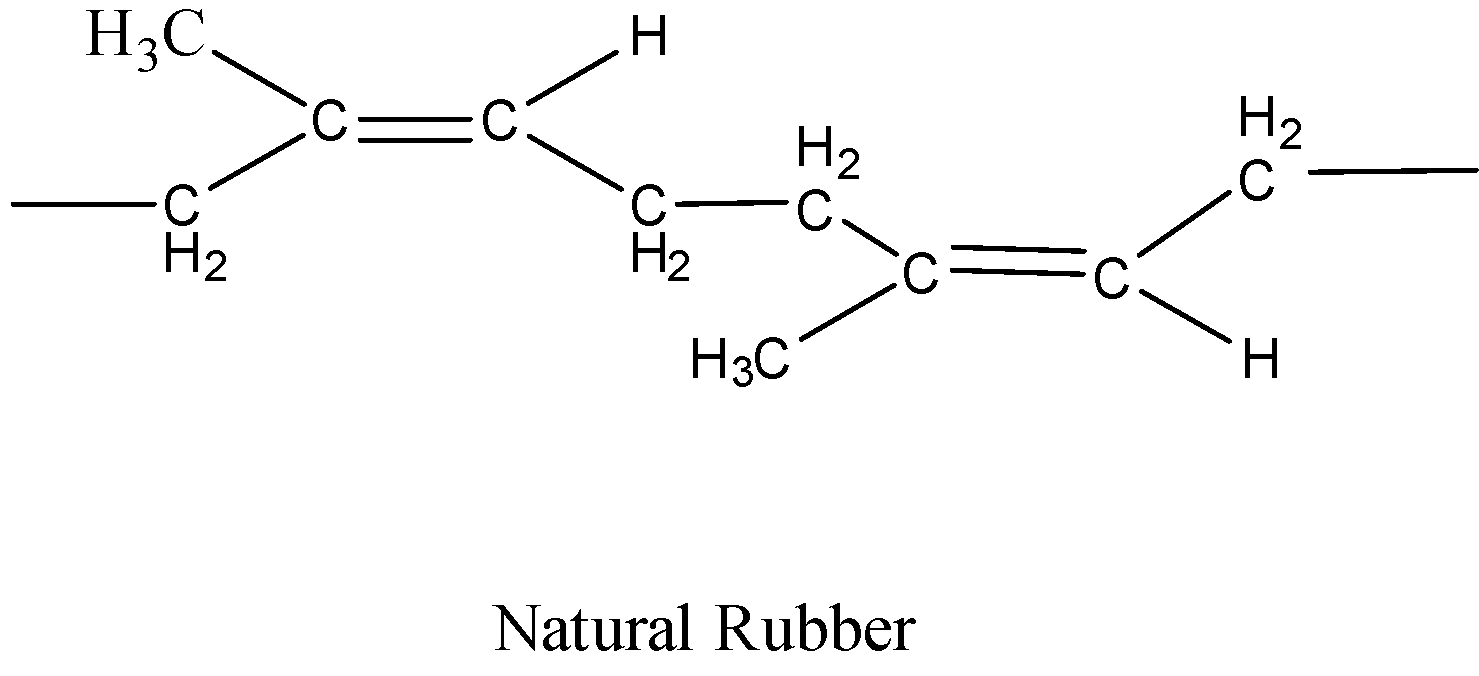
We can see that the monomer unit of this polymer is a 5 carbon compound because it is repeated in the structure of the polymer. The monomer will be having double bonds at the terminal carbons because they will make bonds with the other molecules of the monomer.
- Now, let’s draw the structure of the monomer we can see and then we will name it.
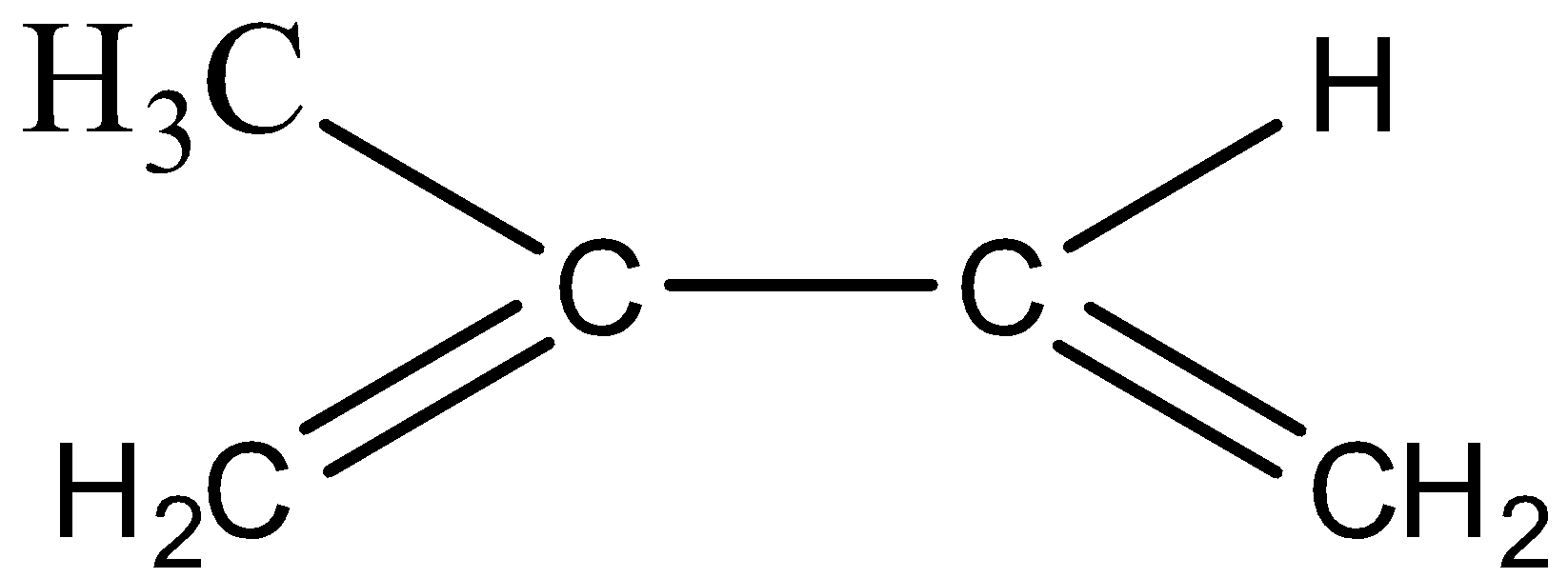
- The molecule given above is the monomer of natural rubber and its IUPAC name will be 2-methyl-1,3-butadiene.
- 2-methyl-1,3-butadiene is also called cis-Isoprene and the polymer natural rubber is also named as cis-1,4-polyisoprene.
- The structure of natural rubber consists of various chains of polymer and they are held together by van der Waals force.
Additional Information:
- Rubber is a natural polymer.
- It possesses elastic properties, so it is also called elastomer as well.
- Natural rubber is also known as Latex.
- It is a liner type of polymer as no branching in polymerization occurs in it.
Note: Remember that trans-poly isoprene is not a Natural rubber, only cis-poly isoprene is natural rubber. If you know the structure of the isomer, then be careful while naming it i.e. methyl group containing carbon is given number-2 and not number-3.
Complete step by step solution:
Natural rubber has the structure as shown below.

We can see that the monomer unit of this polymer is a 5 carbon compound because it is repeated in the structure of the polymer. The monomer will be having double bonds at the terminal carbons because they will make bonds with the other molecules of the monomer.
- Now, let’s draw the structure of the monomer we can see and then we will name it.

- The molecule given above is the monomer of natural rubber and its IUPAC name will be 2-methyl-1,3-butadiene.
- 2-methyl-1,3-butadiene is also called cis-Isoprene and the polymer natural rubber is also named as cis-1,4-polyisoprene.
- The structure of natural rubber consists of various chains of polymer and they are held together by van der Waals force.
Additional Information:
- Rubber is a natural polymer.
- It possesses elastic properties, so it is also called elastomer as well.
- Natural rubber is also known as Latex.
- It is a liner type of polymer as no branching in polymerization occurs in it.
Note: Remember that trans-poly isoprene is not a Natural rubber, only cis-poly isoprene is natural rubber. If you know the structure of the isomer, then be careful while naming it i.e. methyl group containing carbon is given number-2 and not number-3.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




