
Name the following compounds
A.
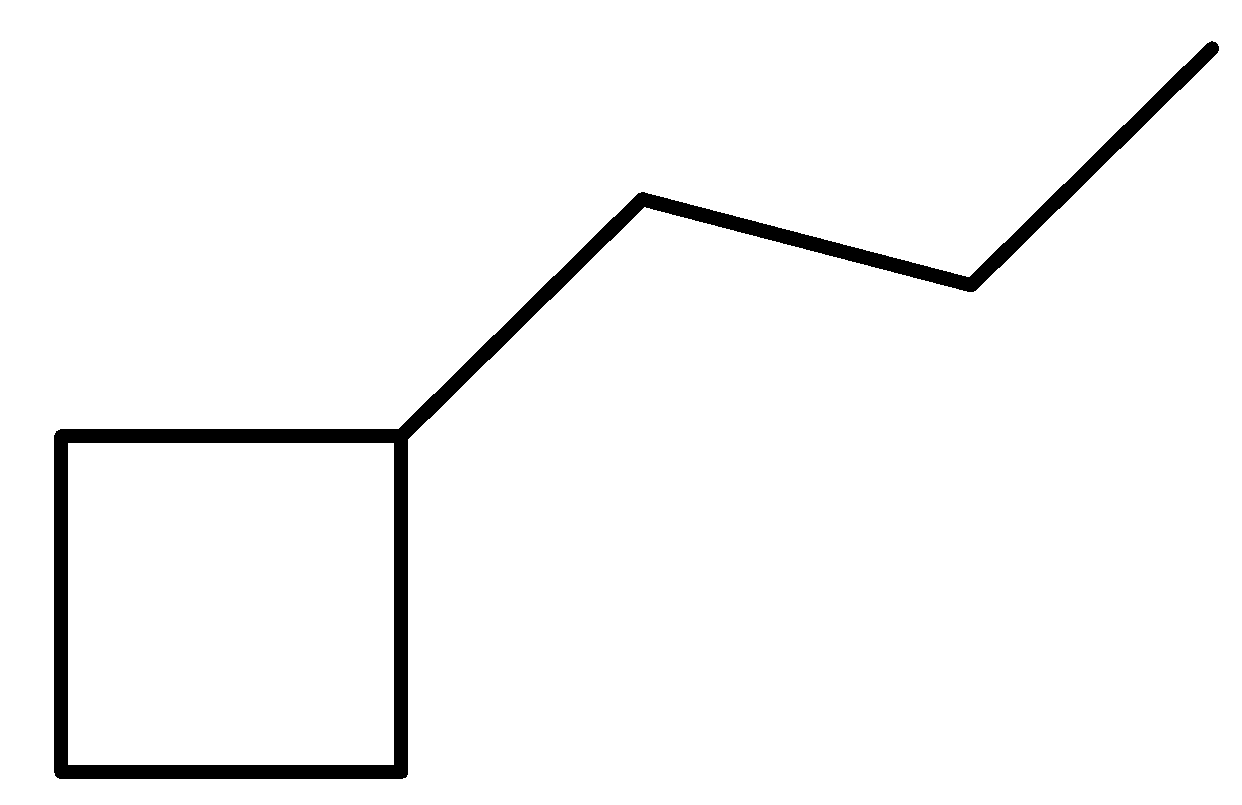
B.
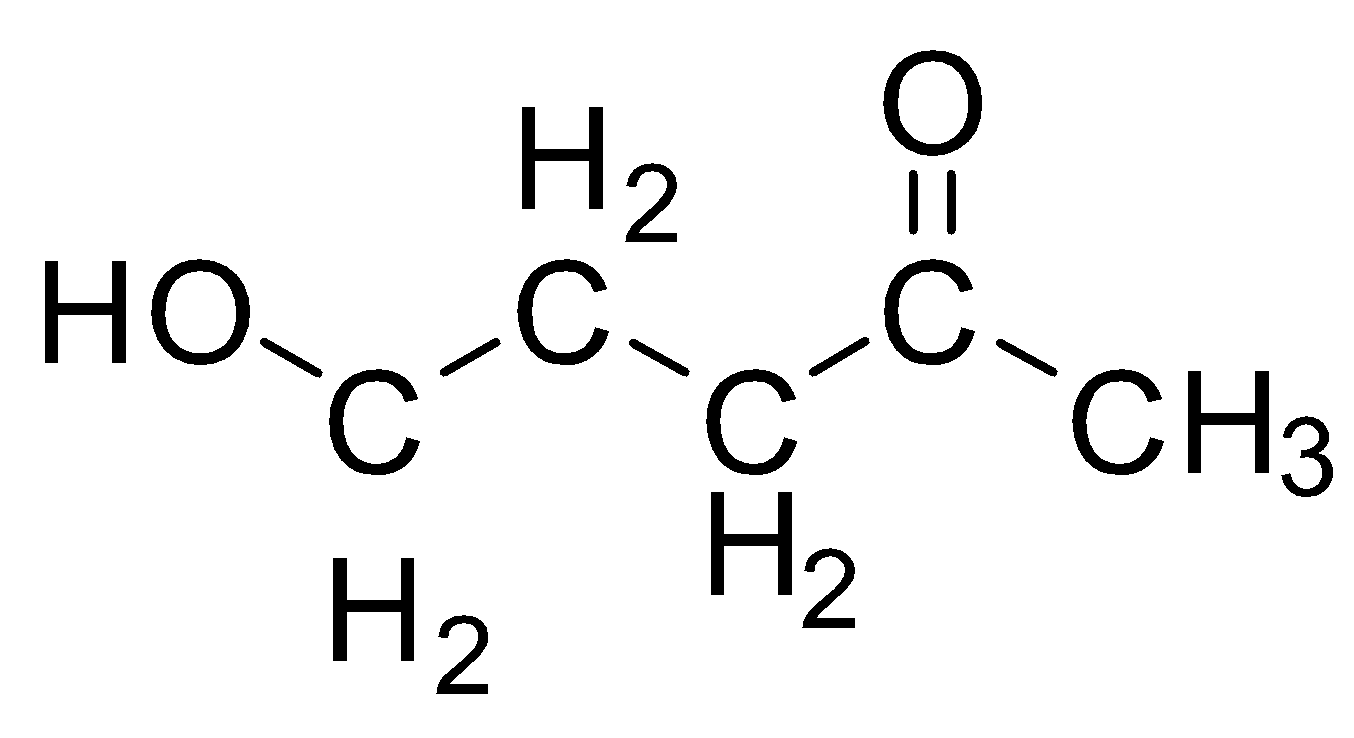
C.
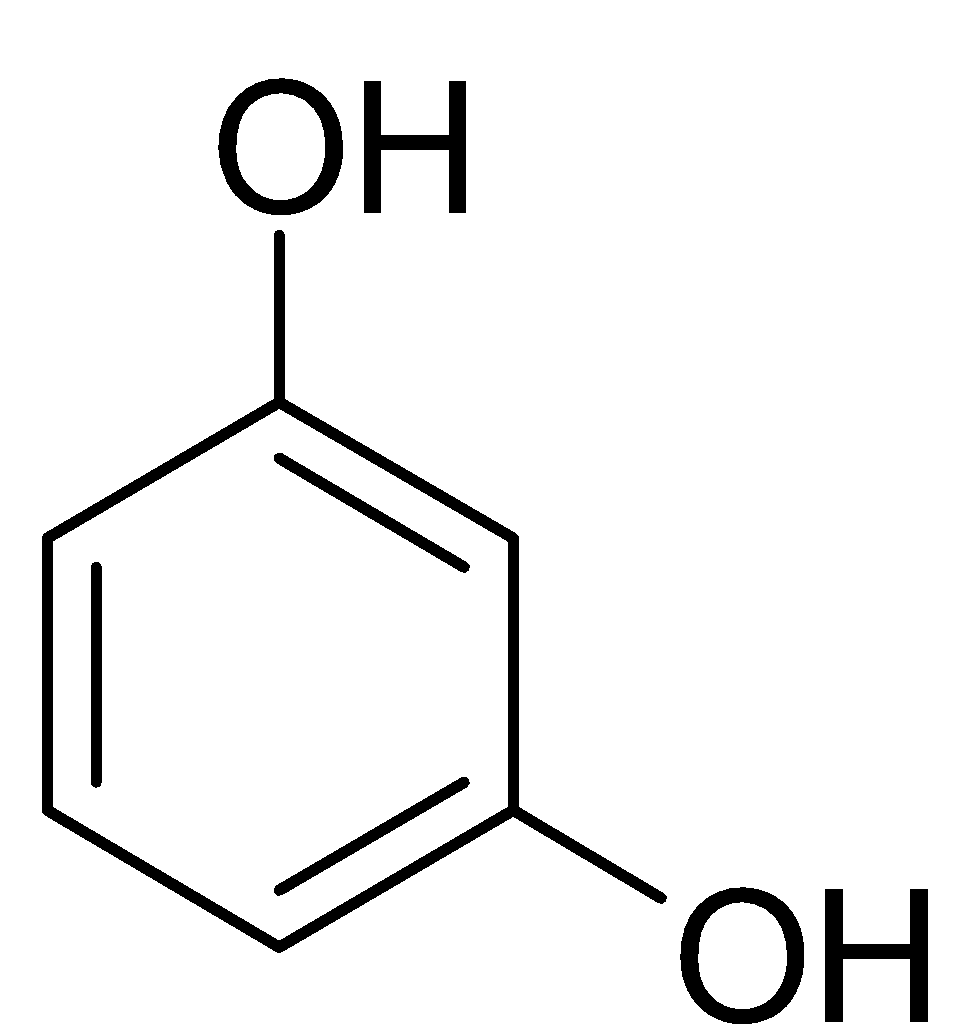



Answer
573.6k+ views
Hint: Use these rules to name the compound
1. In a compound where cyclic group has a alkyl substituent, the ring becomes the parent chain and is written as cyclo + number of carbon chain
2. In a molecule with carbonyl and hydroxyl substituents, carbonyl groups are given preference. The carbonyl name is used in the parent chain and the hydroxyl group is mentioned as a prefix.
3. In any aromatic compound, such as compounds with benzene, the benzene ring acts as the parent chain and secondary groups are mentioned as their prefix.
Complete step by step answer:
1.
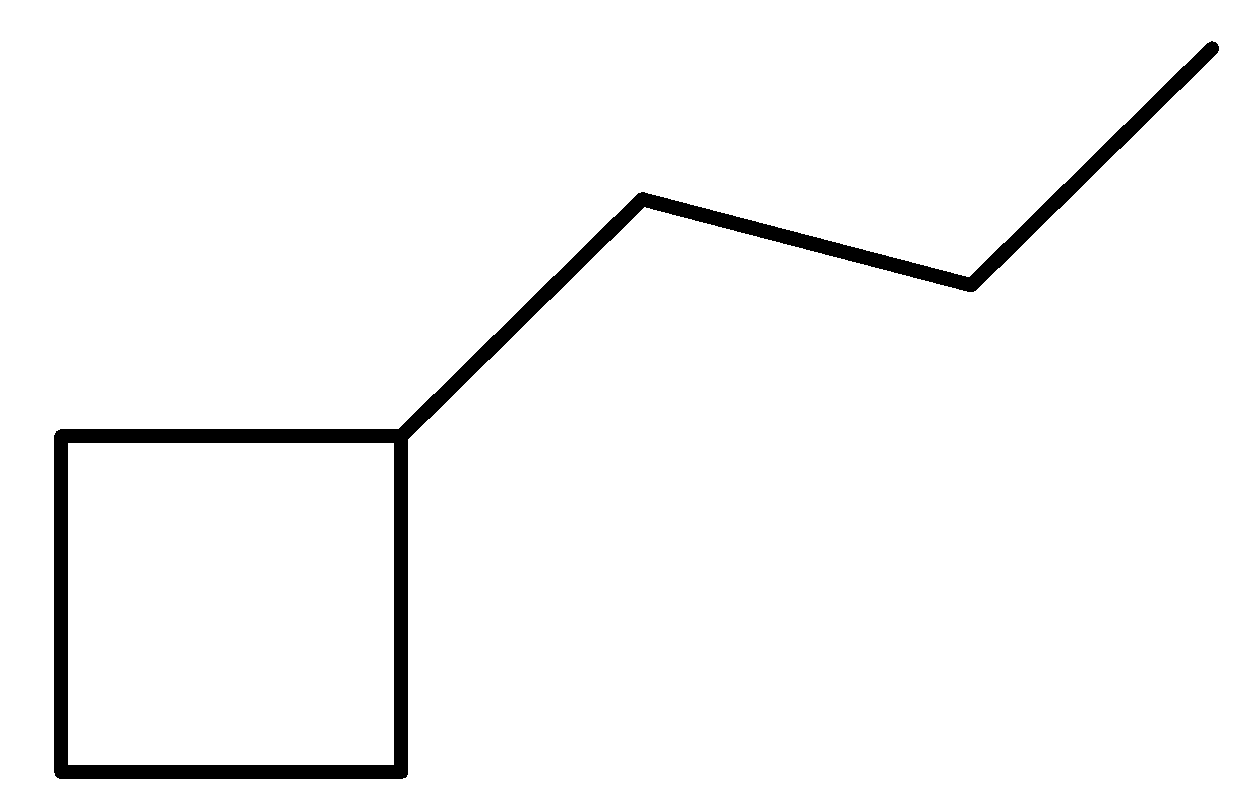
Let us break down the compound into two simpler parts for naming purposes. First, we have a ring structure made of four carbon and a linear chain made up of three carbons. A simple Hydrocarbon chain with four carbon is called butane. In case of a cyclic butane structure, we use the prefix cyclo in front of the parent chain to indicate the presence of a ring. The side chain has three carbon and will be called propyl as it’s a side chain substituent.
The name of this compound is: Propyl Cyclobutane
2.
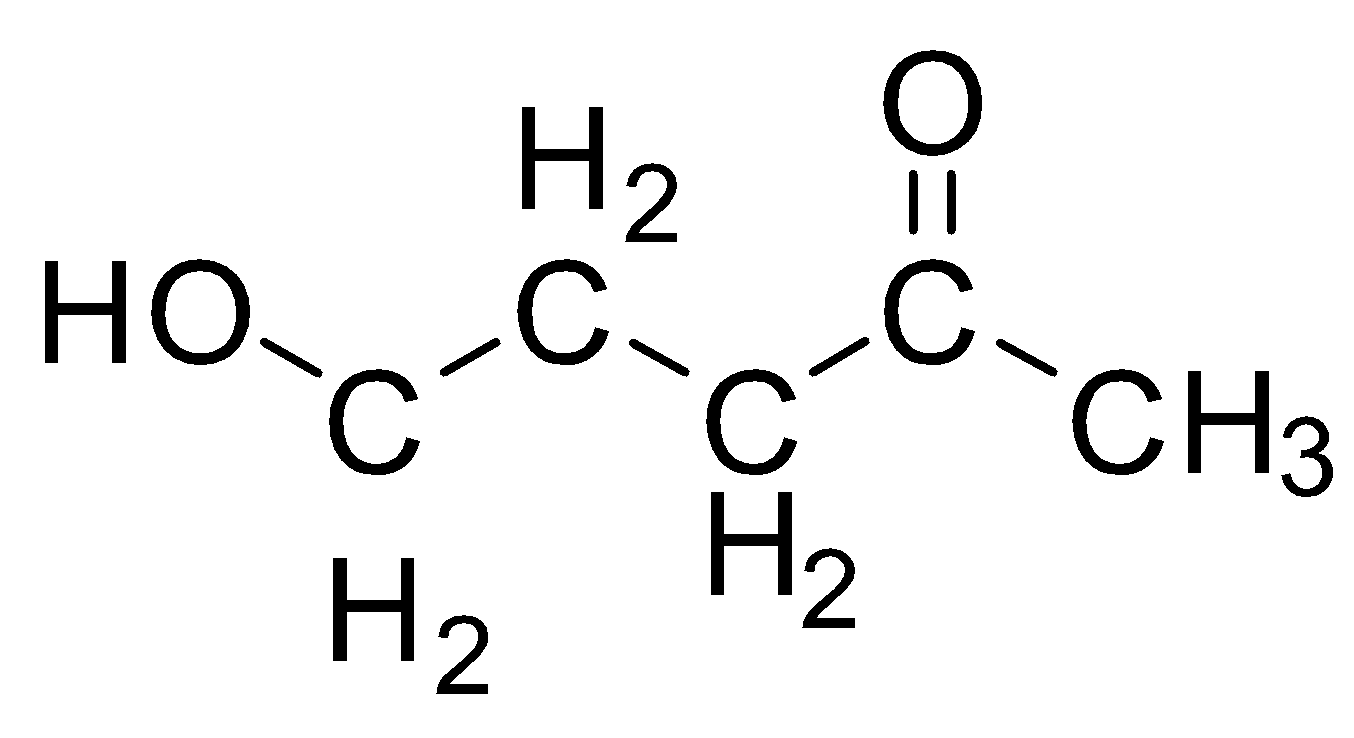
We have a linear chain of five carbons in this structure and hence the parent name will be a derivative of pentane. Now, we have two substituents, one is the ketone group on the second carbon, the second substituent is the alcohol group on the fifth carbon. Numbering will start in such a way to fit maximum possible carbon in the chain but also, so that the primary functional group, which in this case is the ketone, should have the least number. Hence Number starts from $C{H_3}$ carbon as one and not from $C{H_2} - OH$ carbon. so the alcohol group is a secondary group and is mentioned before the parent group as a prefix. The prefix for hydroxy. When we have ketone as a primary functional group, we substitute the “e” from parent alkane with “one” The name of this compound is: $5-hydroxypentan-2-one$ 3.
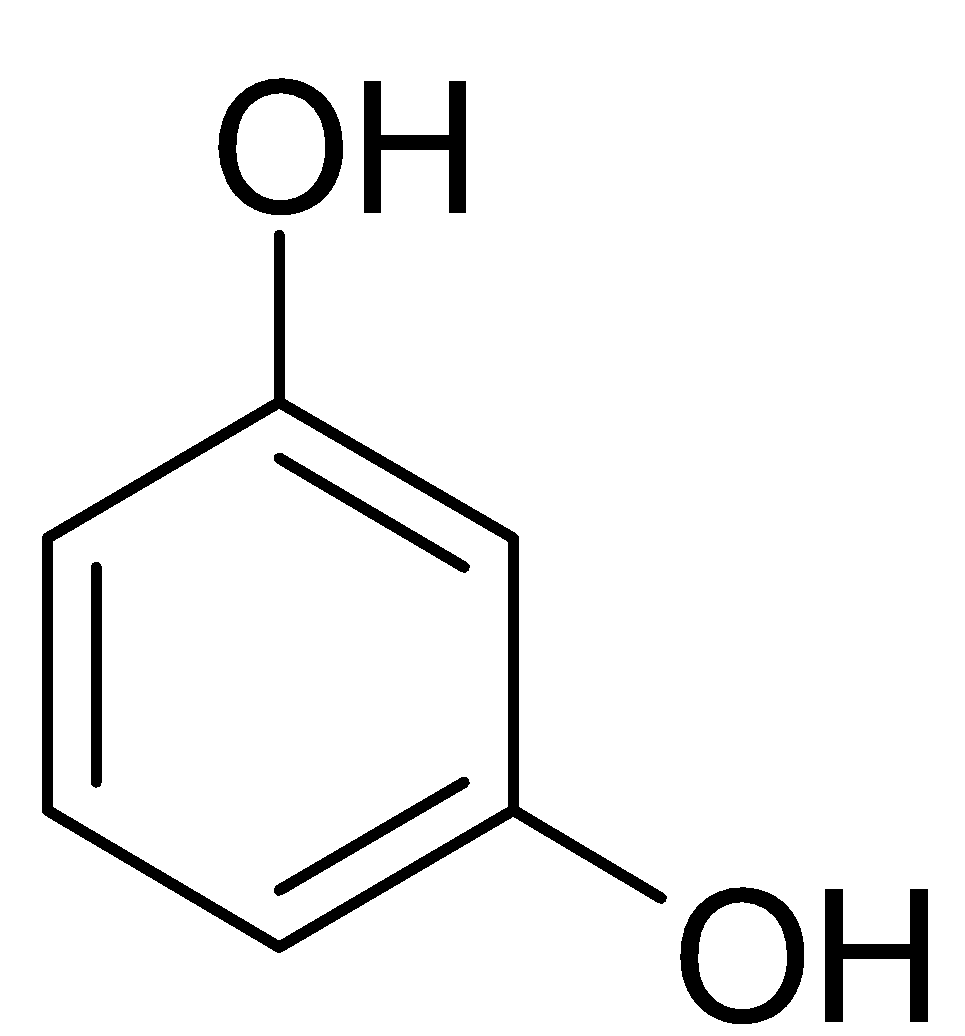
In this molecule, we have an aromatic ring with two hydroxyl groups as substituents. To derive the IUPAC of this structure we will label the carbons from one to six in such a way that the hydroxyl groups will have the least value of carbon number. Hence they will either be $1,3$ alcohol or they will be $1,5$ alcohol. Since, we need least value possible, We use $1,3$ alcohol. When we have alcohol as primary functional group, we substitute the “e” from the parent alkane with “ol” The name of this compound is: $Benzene-1,3-diol$
Note: IUPAC Name stands for International Union of Pure and Applied Chemistry, these are scientific names given to molecules on the basis of their atomic arrangements. However, many chemicals have a common name by which they are widely known. For example: the common name for $Benzene-1,3-diol$ is resorcinol.
1. In a compound where cyclic group has a alkyl substituent, the ring becomes the parent chain and is written as cyclo + number of carbon chain
2. In a molecule with carbonyl and hydroxyl substituents, carbonyl groups are given preference. The carbonyl name is used in the parent chain and the hydroxyl group is mentioned as a prefix.
3. In any aromatic compound, such as compounds with benzene, the benzene ring acts as the parent chain and secondary groups are mentioned as their prefix.
Complete step by step answer:
1.

Let us break down the compound into two simpler parts for naming purposes. First, we have a ring structure made of four carbon and a linear chain made up of three carbons. A simple Hydrocarbon chain with four carbon is called butane. In case of a cyclic butane structure, we use the prefix cyclo in front of the parent chain to indicate the presence of a ring. The side chain has three carbon and will be called propyl as it’s a side chain substituent.
The name of this compound is: Propyl Cyclobutane
2.

We have a linear chain of five carbons in this structure and hence the parent name will be a derivative of pentane. Now, we have two substituents, one is the ketone group on the second carbon, the second substituent is the alcohol group on the fifth carbon. Numbering will start in such a way to fit maximum possible carbon in the chain but also, so that the primary functional group, which in this case is the ketone, should have the least number. Hence Number starts from $C{H_3}$ carbon as one and not from $C{H_2} - OH$ carbon. so the alcohol group is a secondary group and is mentioned before the parent group as a prefix. The prefix for hydroxy. When we have ketone as a primary functional group, we substitute the “e” from parent alkane with “one” The name of this compound is: $5-hydroxypentan-2-one$ 3.

In this molecule, we have an aromatic ring with two hydroxyl groups as substituents. To derive the IUPAC of this structure we will label the carbons from one to six in such a way that the hydroxyl groups will have the least value of carbon number. Hence they will either be $1,3$ alcohol or they will be $1,5$ alcohol. Since, we need least value possible, We use $1,3$ alcohol. When we have alcohol as primary functional group, we substitute the “e” from the parent alkane with “ol” The name of this compound is: $Benzene-1,3-diol$
Note: IUPAC Name stands for International Union of Pure and Applied Chemistry, these are scientific names given to molecules on the basis of their atomic arrangements. However, many chemicals have a common name by which they are widely known. For example: the common name for $Benzene-1,3-diol$ is resorcinol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




