
Name the following;
1.The non- sublimable solid from a mixture of iodine and potassium nitrate.
2.The heavier liquid component from – mercury and water
3.The lower boiling point component from methyl alcohol and water.
4.The compound containing one atom of Sulphur and two atoms of oxygen
5.An acid whose formula is \[{H_2}C{O_3}\]
Answer
564.9k+ views
Hint:Solids undergo sublimation at low pressure. However, few solids undergo sublimation at atmospheric temperature and pressure.
The heavier liquid has a high density and with this we can identify the answer.
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point increases with the molecular mass.
Carbonic acid has two acyl groups bonded to the same oxygen atom.
Complete step by step answer:
Let us discuss each given part in detail.
1.A mixture of iodine and potassium nitrate
-Sublimable solid – The solid compounds which undergo sublimation are called sublimable solid.
-Non – sublimable – Those solid compounds which directly on heating without passing through the liquid phase are called non – sublimable compounds.
-Iodine is a sublimable solid. Thus Potassium nitrate is a non-sublimable solid.
-The correct answer is Potassium nitrate.
2.Heavier liquid component
Mercury has a density of $(13.5\,gm{L^{ - 1}})$ , which is 13.5 times denser than water $\left( {1.0gm{L^{ - 1}}} \right)$, so a small amount of mercury like this feels heavy.
-Mercury also has very high surface tension.
-Thus water has a lighter liquid component and mercury has a heavier liquid component.
-The correct answer is Mercury.
3.Lower boiling point component
-Methyl alcohol boiling point is $64.7^\circ C$ $\left( {143.7^\circ F} \right)$
-Water boiling point is $100^\circ C$
-From this methyl alcohol has a lower boiling point than water.
-The correct answer is Methyl alcohol.
4.The compound contains one atom of Sulphur and two-atom of oxygen
Sulphur dioxide is the chemical compound with the formula $S{O_2}$. The structure of Sulphur dioxide is as follows:
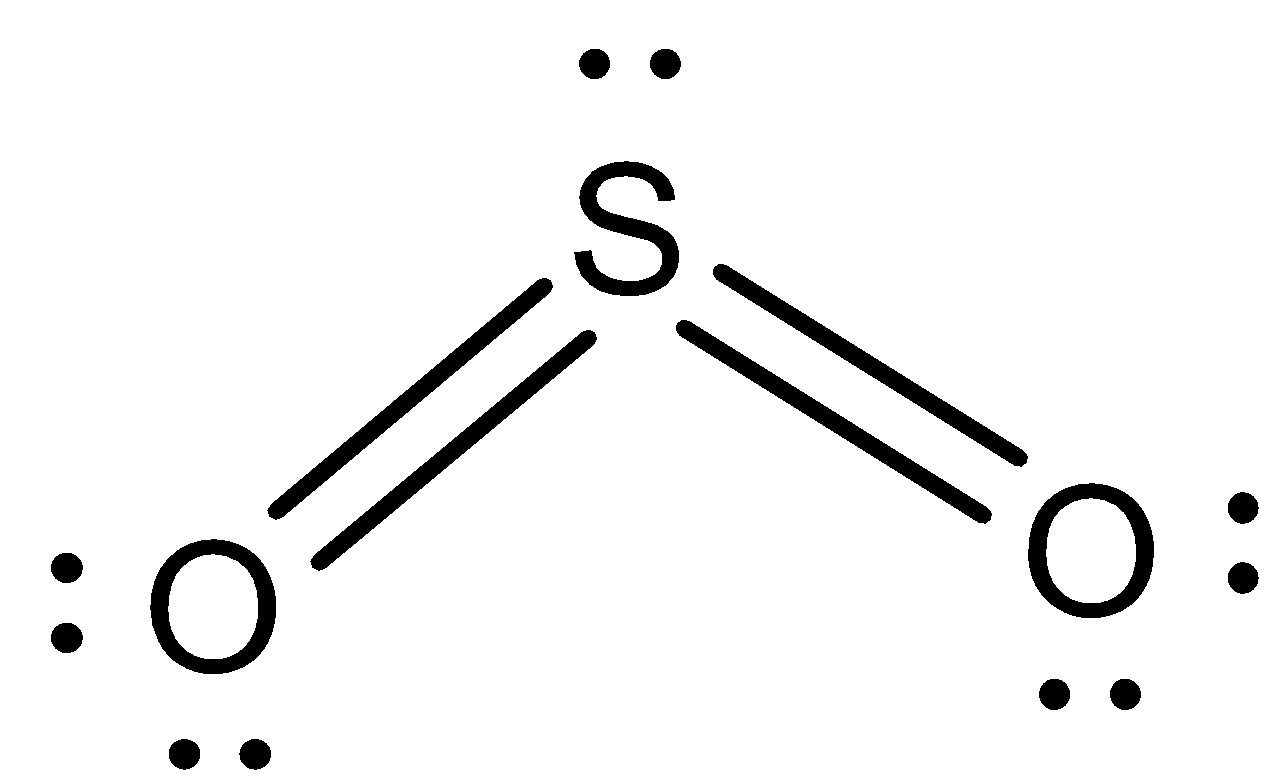
The correct answer is Sulphur dioxide.
5.Acid formula is ${H_2}C{O_3}$
Carbonic acid (${H_2}C{O_3}$) , is the carbon chemical compound whose acid formula is ${H_2}C{O_3}$ . The structure of carbonic acid is as follows:
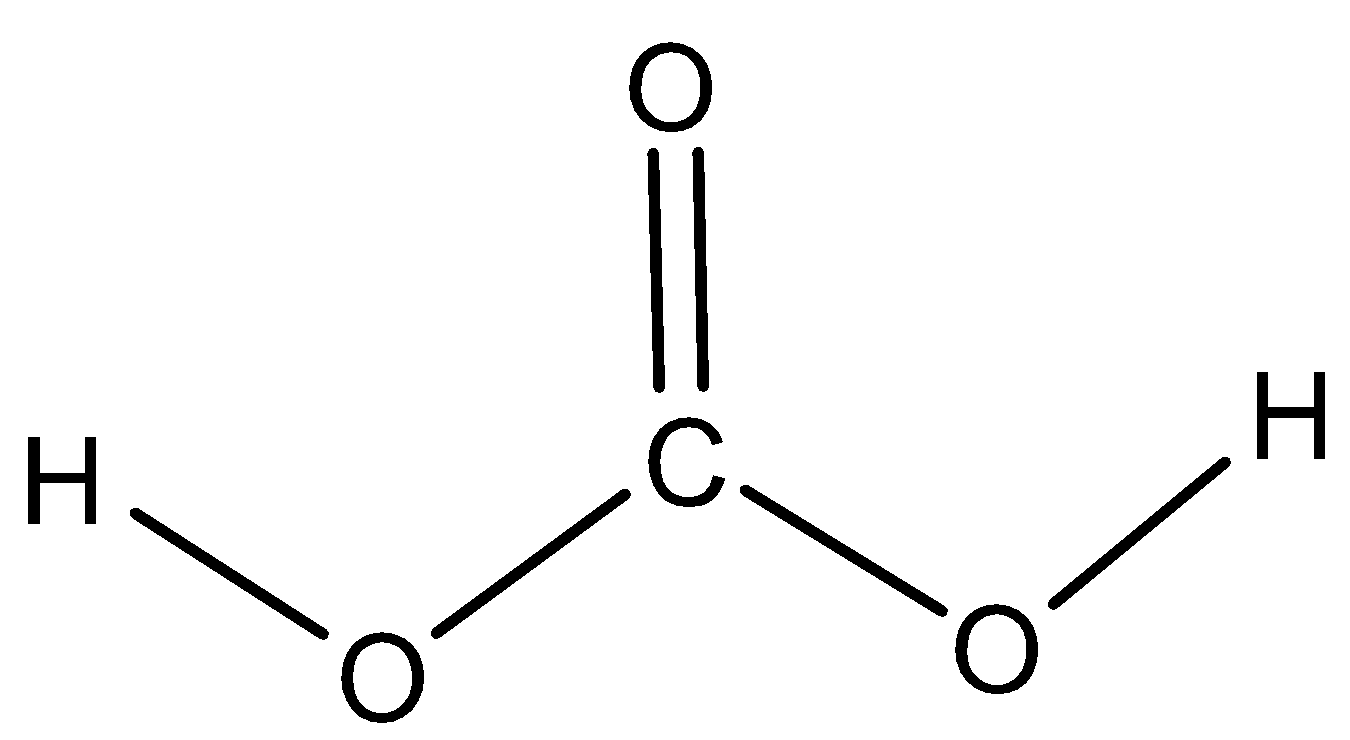
The correct answer is Carbonic acid.
Note:
Iodine can be heated and undergo sublimation. It does not form a liquid under standard pressure.
In the above question, mercury is denser than water. With this, we can identify the answer. In this density of the liquid is a key matter.
The vapor pressure increases with temperature because, at a higher temperature, the molecule moves faster and has a higher tendency to overcome attractive intermolecular forces.
Carbonic acid helps in the transportation of carbon dioxide out of the body. It can be consumed orally to induce vomiting whenever required (such as in drug overdose cases).
The heavier liquid has a high density and with this we can identify the answer.
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point increases with the molecular mass.
Carbonic acid has two acyl groups bonded to the same oxygen atom.
Complete step by step answer:
Let us discuss each given part in detail.
1.A mixture of iodine and potassium nitrate
-Sublimable solid – The solid compounds which undergo sublimation are called sublimable solid.
-Non – sublimable – Those solid compounds which directly on heating without passing through the liquid phase are called non – sublimable compounds.
-Iodine is a sublimable solid. Thus Potassium nitrate is a non-sublimable solid.
-The correct answer is Potassium nitrate.
2.Heavier liquid component
Mercury has a density of $(13.5\,gm{L^{ - 1}})$ , which is 13.5 times denser than water $\left( {1.0gm{L^{ - 1}}} \right)$, so a small amount of mercury like this feels heavy.
-Mercury also has very high surface tension.
-Thus water has a lighter liquid component and mercury has a heavier liquid component.
-The correct answer is Mercury.
3.Lower boiling point component
-Methyl alcohol boiling point is $64.7^\circ C$ $\left( {143.7^\circ F} \right)$
-Water boiling point is $100^\circ C$
-From this methyl alcohol has a lower boiling point than water.
-The correct answer is Methyl alcohol.
4.The compound contains one atom of Sulphur and two-atom of oxygen
Sulphur dioxide is the chemical compound with the formula $S{O_2}$. The structure of Sulphur dioxide is as follows:

The correct answer is Sulphur dioxide.
5.Acid formula is ${H_2}C{O_3}$
Carbonic acid (${H_2}C{O_3}$) , is the carbon chemical compound whose acid formula is ${H_2}C{O_3}$ . The structure of carbonic acid is as follows:

The correct answer is Carbonic acid.
Note:
Iodine can be heated and undergo sublimation. It does not form a liquid under standard pressure.
In the above question, mercury is denser than water. With this, we can identify the answer. In this density of the liquid is a key matter.
The vapor pressure increases with temperature because, at a higher temperature, the molecule moves faster and has a higher tendency to overcome attractive intermolecular forces.
Carbonic acid helps in the transportation of carbon dioxide out of the body. It can be consumed orally to induce vomiting whenever required (such as in drug overdose cases).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




