
Name A, B, C, D, E and F in the following diagram showing change of state:
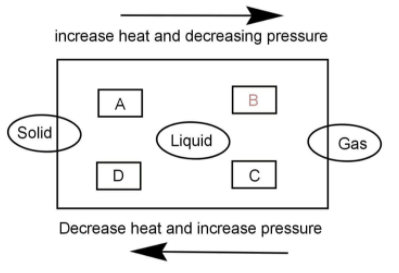
A) A = Melting, B = Vaporisation, C = Condensation, D = Solidification, E = Sublimation, F = solidification of gaseous state.
B) A = Sublimation, B = Vaporisation, C = Condensation, D = Solidification, E = Melting, F = solidification of gaseous state.
C) A = Condensation, B = Vaporisation, C = Melting, D = Solidification, E = Sublimation, F = solidification of gaseous state.
D) A = Vaporisation, B = Melting, C = Condensation, D = Solidification, E = Sublimation, F = solidification of gaseous state.

Answer
558k+ views
Hint:
The conversion of solid to liquid is termed as melting and the reverse process is called freezing. The conversion of liquid to gas is called vaporization and the reverse is known as condensation. The conversion of solid to gas is known as sublimation.
Complete step by step solution:
Let us look at what type of conversion each of the processes is doing.
In the process A solid is being converted into liquid. Whenever solid converts to liquid the process is known as melting.
The process A is melting.
In the process B Liquid is being converted into gas. The conversion of liquid to gas is known as vaporization and hence,
The process B is vaporisation.
Now coming to process C, this is the reverse of process B that is now the gas is converted into liquid which is known as condensation.
The process C is condensation.
Process D is reverse of melting, in this the liquid converts into solid and this is known as freezing or solidification.
The process D is solidification.
The process E is conversion of solid into gas this is called the sublimation of gas that is solid sublimes into gas.
The process E is sublimation.
The conversion of gas to solid is very obvious to be called solidification of gaseous state.
According to the above points, the correct option is A.
Note:
Solid, liquid and gas are the three states of matter. Solids have maximum force of attraction and gases have least. Whenever the temperature is increased and pressure is reduced then the intermolecular force of attraction decreases. When we increase temperature or decrease pressure in solid state it tends to move where intermolecular force decreases that is to the liquid state and the reverse is also true.
The conversion of solid to liquid is termed as melting and the reverse process is called freezing. The conversion of liquid to gas is called vaporization and the reverse is known as condensation. The conversion of solid to gas is known as sublimation.
Complete step by step solution:
Let us look at what type of conversion each of the processes is doing.
In the process A solid is being converted into liquid. Whenever solid converts to liquid the process is known as melting.
The process A is melting.
In the process B Liquid is being converted into gas. The conversion of liquid to gas is known as vaporization and hence,
The process B is vaporisation.
Now coming to process C, this is the reverse of process B that is now the gas is converted into liquid which is known as condensation.
The process C is condensation.
Process D is reverse of melting, in this the liquid converts into solid and this is known as freezing or solidification.
The process D is solidification.
The process E is conversion of solid into gas this is called the sublimation of gas that is solid sublimes into gas.
The process E is sublimation.
The conversion of gas to solid is very obvious to be called solidification of gaseous state.
According to the above points, the correct option is A.
Note:
Solid, liquid and gas are the three states of matter. Solids have maximum force of attraction and gases have least. Whenever the temperature is increased and pressure is reduced then the intermolecular force of attraction decreases. When we increase temperature or decrease pressure in solid state it tends to move where intermolecular force decreases that is to the liquid state and the reverse is also true.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

How was the Civil Disobedience Movement different from class 12 social science CBSE




