
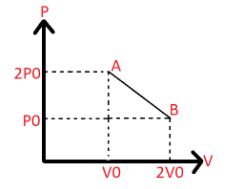
$'n'$ moles of an ideal gas undergoes a process $A \to B$ as shown in the figure. The maximum temperature of the gas during the process will be:
A. $\dfrac{{3{P_0}{V_0}}}{{2nR}}$
B. $\dfrac{{9{P_0}{V_0}}}{{2nR}}$
C. $\dfrac{{9{P_0}{V_0}}}{{nR}}$
D. $\dfrac{{9{P_0}{V_0}}}{{4nR}}$

Answer
573.6k+ views
Hint:After establishing a relationship between the two variables of pressure and volume using the equation of a straight line pertaining to the graph, we will equate it with the ideal gas equation. Using the boundary conditions for maximum temperature, we will be able to determine the maximum temperature.
Formulae used:
Equation of a straight line: $(x - {x_0}) = \dfrac{{{x_2} - {x_1}}}{{{y_2} - {y_1}}}(y - {y_0})$
Where $y,{y_1},{y_2}$ and $x,{x_1},{x_2}$ are the y and x intercepts of a graph.
Ideal gas equation: $PV = nRT$
Where $P$ is the pressure of the gas and is expressed in Bars $(B)$, $V$ is the volume of the gas and is expressed in meter cube $({m^3})$, $n$ is the number of moles of the gas, $R$ is the universal gas constant and $T$ is the temperature of the gas and is expressed in Celsius $(^\circ C)$.
Complete step by step answer:
From the graph we have the following data:
At A:
Pressure $ = 2{P_0}$
Volume $ = {V_0}$
At B:
Pressure $ = {P_0}$
Volume $ = 2{V_0}$
To establish a relationship between the two, we will use the equation of a line for the given graph.
We know that, $(y - {y_0}) = \dfrac{{{y_2} - {y_1}}}{{{x_2} - {x_1}}}(x - {x_0})$.
Substituting the intercepts according to our graph we get,
$(V - {V_0}) = \dfrac{{{V_2} - {V_1}}}{{{P_2} - {P_1}}}(P - {P_0})$
According to the graph, \[{V_2} = 2{V_0},{V_1} = {V_0},{P_2} = {P_0},{P_1} = 2{P_0}\]. Substituting the same we get,
$(V - {V_0}) = \dfrac{{2{V_0} - {V_0}}}{{{P_0} - 2{P_0}}}(P - {P_0})$
Simplifying the above equation we get,
\[
(V - {V_0}) = \dfrac{{{V_0}}}{{ - {P_0}}}(P - {P_0}) \\
\Rightarrow (P - {P_0}) = (V - {V_0})\dfrac{{ - {P_0}}}{{{V_0}}} \\
\Rightarrow (P - {P_0}) = \dfrac{{ - {P_0}V}}{{{V_0}}} + 2{P_0} \\
\Rightarrow P = \dfrac{{ - {P_0}V}}{{{V_0}}} + 2{P_0} + {P_0} \\
\Rightarrow P = \dfrac{{ - {P_0}}}{{{V_0}}}(V - 3{V_0}) \\
\]
Now, we know that for an ideal gas $PV = nRT$
Rearranging this we get,
$P = \dfrac{{nRT}}{V}$
Substituting the above in the relationship established between volume and pressure we get,
$\dfrac{{nRT}}{V} = \dfrac{{ - {P_0}}}{{{V_0}}}(V - 3{V_0})$
Upon rearrangement to arrive at an expression for temperature $T$ we get,
$T = \dfrac{{ - {P_0}}}{{nR{V_0}}}({V^2} - 3{V_0}V)$
We need to determine the value of $V$.
Now, we know that at maximum temperature, rate of change of temperature with respect to volume of an ideal gas is constant. That is $\dfrac{{dT}}{{dV}} = 0$.
Therefore, differentiating the expression for temperature with respect to volume we get,
$\dfrac{{dT}}{{dV}} = \dfrac{{d(\dfrac{{ - {P_0}}}{{nR{V_0}}}({V^2} - 3{V_0}V))}}{{dV}}$
Upon simplification and substitution of boundary condition $\dfrac{{dT}}{{dV}} = 0$ we will establish a relationship between volume and pressure only,
$
\dfrac{{dT}}{{dV}} = \dfrac{{d(\dfrac{{ - {P_0}}}{{nR{V_0}}}({V^2} - 3{V_0}V))}}{{dV}} \\
\Rightarrow 0 = \dfrac{{d(\dfrac{{ - {P_0}}}{{nR{V_0}}}({V^2} - 3{V_0}V))}}{{dV}} \\
\Rightarrow 0 = \dfrac{{ - {P_0}}}{{{V_0}}}({V^2} - 3{V_0}V) \\
\Rightarrow 3{P_0} = \dfrac{{{P_0}}}{{{V_0}}}2V \\
\Rightarrow 2V - 3{V_0} = 0 \\
\Rightarrow V = \dfrac{{3{V_0}}}{2} \\
$
Substituting this value in the equation for temperature we get,
$
T = \dfrac{{ - {P_0}}}{{nR{V_0}}}({V^2} - 3{V_0}V) \\
\Rightarrow T = \dfrac{{ -{P_0}}}{{nR{V_0}}}(\dfrac{9}{4}{V_0}^2 - \dfrac{9}{2}{V_0}^2) \\
\Rightarrow T = \dfrac{{ -{P_0}}}{{nR{V_0}}}\dfrac{{ - 9}}{4}{V_0}^2 \\
\therefore T = \dfrac{{9{P_0}{V_0}}}{{4nR}} \\
$
Therefore, The maximum temperature of the gas during the process will be $\dfrac{{9{P_0}{V_0}}}{{4nR}}$.
Hence, the correct option is D.
Note: All assumptions are made considering the gas in question as an ideal gas. For real gases equations and relations become more complex and difficult to calculate. Therefore assuming gas to be an ideal gas is the easiest way to solve the question.
Formulae used:
Equation of a straight line: $(x - {x_0}) = \dfrac{{{x_2} - {x_1}}}{{{y_2} - {y_1}}}(y - {y_0})$
Where $y,{y_1},{y_2}$ and $x,{x_1},{x_2}$ are the y and x intercepts of a graph.
Ideal gas equation: $PV = nRT$
Where $P$ is the pressure of the gas and is expressed in Bars $(B)$, $V$ is the volume of the gas and is expressed in meter cube $({m^3})$, $n$ is the number of moles of the gas, $R$ is the universal gas constant and $T$ is the temperature of the gas and is expressed in Celsius $(^\circ C)$.
Complete step by step answer:
From the graph we have the following data:
At A:
Pressure $ = 2{P_0}$
Volume $ = {V_0}$
At B:
Pressure $ = {P_0}$
Volume $ = 2{V_0}$
To establish a relationship between the two, we will use the equation of a line for the given graph.
We know that, $(y - {y_0}) = \dfrac{{{y_2} - {y_1}}}{{{x_2} - {x_1}}}(x - {x_0})$.
Substituting the intercepts according to our graph we get,
$(V - {V_0}) = \dfrac{{{V_2} - {V_1}}}{{{P_2} - {P_1}}}(P - {P_0})$
According to the graph, \[{V_2} = 2{V_0},{V_1} = {V_0},{P_2} = {P_0},{P_1} = 2{P_0}\]. Substituting the same we get,
$(V - {V_0}) = \dfrac{{2{V_0} - {V_0}}}{{{P_0} - 2{P_0}}}(P - {P_0})$
Simplifying the above equation we get,
\[
(V - {V_0}) = \dfrac{{{V_0}}}{{ - {P_0}}}(P - {P_0}) \\
\Rightarrow (P - {P_0}) = (V - {V_0})\dfrac{{ - {P_0}}}{{{V_0}}} \\
\Rightarrow (P - {P_0}) = \dfrac{{ - {P_0}V}}{{{V_0}}} + 2{P_0} \\
\Rightarrow P = \dfrac{{ - {P_0}V}}{{{V_0}}} + 2{P_0} + {P_0} \\
\Rightarrow P = \dfrac{{ - {P_0}}}{{{V_0}}}(V - 3{V_0}) \\
\]
Now, we know that for an ideal gas $PV = nRT$
Rearranging this we get,
$P = \dfrac{{nRT}}{V}$
Substituting the above in the relationship established between volume and pressure we get,
$\dfrac{{nRT}}{V} = \dfrac{{ - {P_0}}}{{{V_0}}}(V - 3{V_0})$
Upon rearrangement to arrive at an expression for temperature $T$ we get,
$T = \dfrac{{ - {P_0}}}{{nR{V_0}}}({V^2} - 3{V_0}V)$
We need to determine the value of $V$.
Now, we know that at maximum temperature, rate of change of temperature with respect to volume of an ideal gas is constant. That is $\dfrac{{dT}}{{dV}} = 0$.
Therefore, differentiating the expression for temperature with respect to volume we get,
$\dfrac{{dT}}{{dV}} = \dfrac{{d(\dfrac{{ - {P_0}}}{{nR{V_0}}}({V^2} - 3{V_0}V))}}{{dV}}$
Upon simplification and substitution of boundary condition $\dfrac{{dT}}{{dV}} = 0$ we will establish a relationship between volume and pressure only,
$
\dfrac{{dT}}{{dV}} = \dfrac{{d(\dfrac{{ - {P_0}}}{{nR{V_0}}}({V^2} - 3{V_0}V))}}{{dV}} \\
\Rightarrow 0 = \dfrac{{d(\dfrac{{ - {P_0}}}{{nR{V_0}}}({V^2} - 3{V_0}V))}}{{dV}} \\
\Rightarrow 0 = \dfrac{{ - {P_0}}}{{{V_0}}}({V^2} - 3{V_0}V) \\
\Rightarrow 3{P_0} = \dfrac{{{P_0}}}{{{V_0}}}2V \\
\Rightarrow 2V - 3{V_0} = 0 \\
\Rightarrow V = \dfrac{{3{V_0}}}{2} \\
$
Substituting this value in the equation for temperature we get,
$
T = \dfrac{{ - {P_0}}}{{nR{V_0}}}({V^2} - 3{V_0}V) \\
\Rightarrow T = \dfrac{{ -{P_0}}}{{nR{V_0}}}(\dfrac{9}{4}{V_0}^2 - \dfrac{9}{2}{V_0}^2) \\
\Rightarrow T = \dfrac{{ -{P_0}}}{{nR{V_0}}}\dfrac{{ - 9}}{4}{V_0}^2 \\
\therefore T = \dfrac{{9{P_0}{V_0}}}{{4nR}} \\
$
Therefore, The maximum temperature of the gas during the process will be $\dfrac{{9{P_0}{V_0}}}{{4nR}}$.
Hence, the correct option is D.
Note: All assumptions are made considering the gas in question as an ideal gas. For real gases equations and relations become more complex and difficult to calculate. Therefore assuming gas to be an ideal gas is the easiest way to solve the question.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




