
What is the molecular structure of $ \left[ Co{{\left( N{{H}_{3}} \right)}_{6}} \right]C{{l}_{3}} $ ?
Answer
518.7k+ views
Hint :We know that the complex given is coordination complex hence while the IUPAC name should be written by using the rules accordingly. While calculating the oxidation number one needs to also consider the charge on the complex. Hybridization and magnetic behaviour can be decided based on the electronic configuration.
Complete Step By Step Answer:
According to valence bond theory, the central metal atom will provide a vacant orbital according to coordination number. This vacant orbital undergoes hybridization and forms a coordinate bond with donor atom or ligand. The pairing of electrons of the central metal atom is possible before hybridization in presence of strong field ligands and complex formation involves that newly available vacant orbital, such complexes are known as inner orbital complexes. On the other hand, pairing will not occur in the presence of weak field ligands and complex formation does not involve such an orbital with unpaired electrons in it, such complexes are known as outer orbital complexes.
Generally, ligands with carbon or nitrogen donors are strong field ligands. The ligands with halogen, sulphur or oxygen donors are weak ligands. Monodentate ligands are those species which form only one coordinate bond with a central metal atom like ammonia and water. Bidentate ligands are those species which form two coordinate bonds with metal.
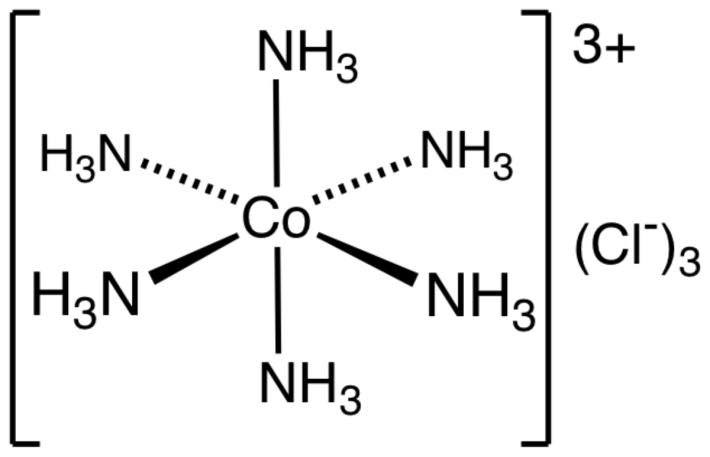
Note :
Remember that the where two bonds are formed from the d-orbital, one from the s-orbital, and three from the p-orbital. The diamagnetic complex is resistant to undergo any change when an external magnetic field is applied. Always remember to consider the charge on the complex while calculating the oxidation state.
Complete Step By Step Answer:
According to valence bond theory, the central metal atom will provide a vacant orbital according to coordination number. This vacant orbital undergoes hybridization and forms a coordinate bond with donor atom or ligand. The pairing of electrons of the central metal atom is possible before hybridization in presence of strong field ligands and complex formation involves that newly available vacant orbital, such complexes are known as inner orbital complexes. On the other hand, pairing will not occur in the presence of weak field ligands and complex formation does not involve such an orbital with unpaired electrons in it, such complexes are known as outer orbital complexes.
Generally, ligands with carbon or nitrogen donors are strong field ligands. The ligands with halogen, sulphur or oxygen donors are weak ligands. Monodentate ligands are those species which form only one coordinate bond with a central metal atom like ammonia and water. Bidentate ligands are those species which form two coordinate bonds with metal.

Note :
Remember that the where two bonds are formed from the d-orbital, one from the s-orbital, and three from the p-orbital. The diamagnetic complex is resistant to undergo any change when an external magnetic field is applied. Always remember to consider the charge on the complex while calculating the oxidation state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




