
Melting point of phenol is:
$A.\;{95^ \circ }C$
$B.\;{43^ \circ }C$
$C.\;{20^ \circ }C$
$D.\;{10^ \circ }C$
Answer
594k+ views
Hint: An organic compound-Phenol is appreciably soluble in water and because of its commercial importance many methods have been developed for its production. They are generally colourless solids or liquids and have relatively low melting point.
Complete answer:
Phenol is also known as carbolic acid or Hydroxybenzene or Phenic acid. And its molecular formula is ${C_6}{H_5}OH$. It is an aromatic organic compound. It is a white crystalline solid that is volatile. The molecule of Phenol consists of a phenyl group $( - {C_6}{H_5})$which is bonded to a hydroxyl group $( - OH)$. It requires careful handling because it is mildly acidic and can cause chemical burns. In aqueous solution the pH range is 8 – 12 pH. And its melting point is ${43^ \circ }C$
Phenol was first extracted from coal tar, but today it is produced on a large scale which is around 7 billion kg/year from petroleum derived feedstocks.
Also, it is used to synthesize plastics and related materials.
The structure of Phenol is
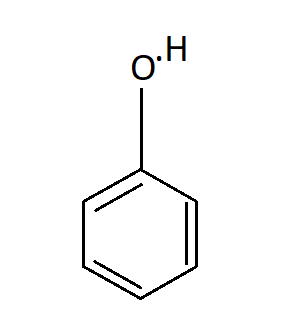
Hence our answer is B. ${43^ \circ }C$
Note: Phenol is a colourless to white solid when pure. It has a distinct odour which is sickeningly sweet and tarry. We can taste and smell phenol at levels lower than those that are associated with harmful effects. It evaporates more slowly than water, and a moderate amount can form a solution with water. Also, they can catch fire.
Complete answer:
Phenol is also known as carbolic acid or Hydroxybenzene or Phenic acid. And its molecular formula is ${C_6}{H_5}OH$. It is an aromatic organic compound. It is a white crystalline solid that is volatile. The molecule of Phenol consists of a phenyl group $( - {C_6}{H_5})$which is bonded to a hydroxyl group $( - OH)$. It requires careful handling because it is mildly acidic and can cause chemical burns. In aqueous solution the pH range is 8 – 12 pH. And its melting point is ${43^ \circ }C$
Phenol was first extracted from coal tar, but today it is produced on a large scale which is around 7 billion kg/year from petroleum derived feedstocks.
Also, it is used to synthesize plastics and related materials.
The structure of Phenol is

Hence our answer is B. ${43^ \circ }C$
Note: Phenol is a colourless to white solid when pure. It has a distinct odour which is sickeningly sweet and tarry. We can taste and smell phenol at levels lower than those that are associated with harmful effects. It evaporates more slowly than water, and a moderate amount can form a solution with water. Also, they can catch fire.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




