
How is the mechanism of reduction of benzophenone to diphenylmethanol?
Answer
559.5k+ views
Hint: Reduction is defined as the loss of electrons. The reduction reaction is the major reaction used to reduce the double bond into single. The benzophenone is reduced to diphenylmethanol using a reducing agent like sodium borohydride.
Complete step by step answer:
The reduction reaction is one of the major classes of reaction used in organic chemistry. Reduction reaction is the opposite of oxidation reaction which is determined by the loss of oxygen from a bond, addition of hydrogen to a bond or replacement of more electronegative atoms by the less electronegative atom. The best method of reduction is hydrogenation reaction which reduces the double bonds into single.
The benzophenone is reduced to diphenylmethanol by using sodium borohydride. The sodium borohydride is used as the reducing agent.
The reaction is shown below.
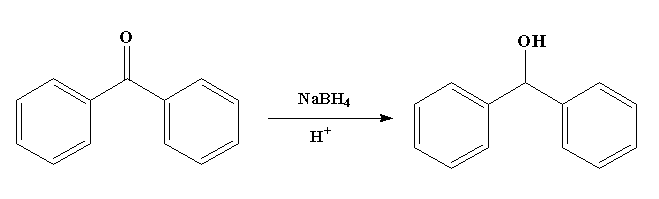
In this reaction, benzophenone is converted to diphenylmethanol with the help of sodium borohydride
The sodium borohydride contains a sodium ion $N{a^ + }$ and borohydride ion $BH_4^ - $. The negative hydride acts as a nucleophile and attacks the carbonyl carbon of the benzophenone and due to this the shifting of bond takes place and the negative charge is generated on the oxygen atom. Then the negative charge of the oxygen attacks and abstract the hydrogen ion to generate hydroxide to form diphenylmethanol.
Note: There is a difference between the hydrogen ion and hydride ion. Hydrogen ion is the cation and hydride ion is the anion. The other reducing agents apart from sodium borohydride is zinc amalgam, Lithium aluminum hydride, diborane, sodium amalgam.
Complete step by step answer:
The reduction reaction is one of the major classes of reaction used in organic chemistry. Reduction reaction is the opposite of oxidation reaction which is determined by the loss of oxygen from a bond, addition of hydrogen to a bond or replacement of more electronegative atoms by the less electronegative atom. The best method of reduction is hydrogenation reaction which reduces the double bonds into single.
The benzophenone is reduced to diphenylmethanol by using sodium borohydride. The sodium borohydride is used as the reducing agent.
The reaction is shown below.

In this reaction, benzophenone is converted to diphenylmethanol with the help of sodium borohydride
The sodium borohydride contains a sodium ion $N{a^ + }$ and borohydride ion $BH_4^ - $. The negative hydride acts as a nucleophile and attacks the carbonyl carbon of the benzophenone and due to this the shifting of bond takes place and the negative charge is generated on the oxygen atom. Then the negative charge of the oxygen attacks and abstract the hydrogen ion to generate hydroxide to form diphenylmethanol.
Note: There is a difference between the hydrogen ion and hydride ion. Hydrogen ion is the cation and hydride ion is the anion. The other reducing agents apart from sodium borohydride is zinc amalgam, Lithium aluminum hydride, diborane, sodium amalgam.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




