
What is the meaning of negative sign in average rate of reaction?
Answer
573.6k+ views
Hint: Process of breakage of old bonds and formation of new bonds is known as chemical reaction. Rate of chemical reaction will depend upon how quickly the breakage of old bonds take place and how quickly the new bonds form . In mathematical terms rate of reaction will actually be the rate of decrease in concentration of reactant or it can also be rate of increase in concentration of product.
Complete step by step solution:
Suppose a reaction is taking place where reactant A is forming a product
- As the reactant will get converted into product, concentration of reactant will decrease and concentration of product will increase.
- Rate of reaction over here will depend upon change in concentration of A. Faster the concentration will decrease: higher will be the rate of reaction.
- Whenever in chemistry we talk, the average rate always remembers that the average rate will always be the change in physical quantity in a time interval which is not very small.
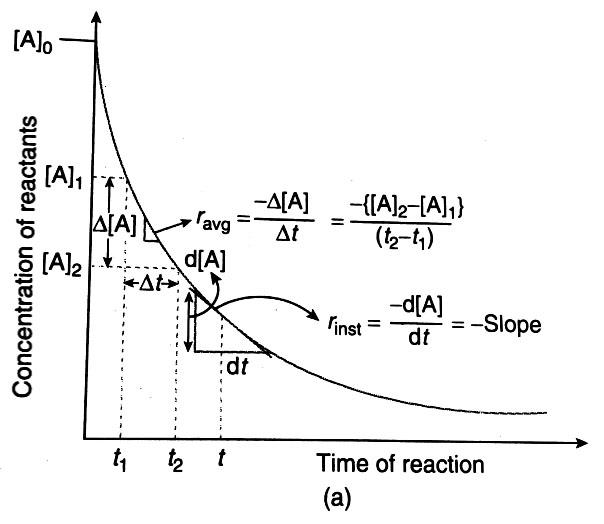
- here we are measuring the concentration of reactant A at two time ${{t}_{1}}$ and time ${{t}_{2}}$ respectively are ${{A}_{1}}$ and${{A}_{2}}$ where ${{A}_{2}}So ratio of change in concentration with change in time will be
$\dfrac{{{A}_{2}}-{{A}_{1}}}{{{t}_{2}}-{{t}_{1}}}$
- Now because concentration of A is decreasing with time and ${{A}_{2}}<{{A}_{1}}$ that’s why this expressing can be written as follow to express the average rate of reaction
$r=-\dfrac{\Delta A}{\Delta t}$
$\Delta A$ is change in concentration and $\Delta t$ is change in time.
- Now change in time is positive but with respect to that change in concentration is negative, so their ratio will be negative but rate of reaction can’t be negative that’s why a negative sign is applied before the expression to keep the rate of reaction positive.
So the significance of negative sign is to show the decrease in concentration of reactant.
- When the time interval $\Delta t$ becomes very small this average rate of reaction becomes an instantaneous rate of reaction.
$r={}_{\Delta t\to 0}\dfrac{-\Delta A}{\Delta t}$
\[r=-\dfrac{dA}{dt}\]
Expression for instantaneous rate of reaction is \[r=-\dfrac{dA}{dt}\]
Note: There is branch of physical chemistry known as chemical kinetics where we study about the rates of different reactions and classify them as fast, moderate and slow reactions based on their rate and we also study about the various factors like temperature, pressure etc. those affect the rate of reaction.
Complete step by step solution:
Suppose a reaction is taking place where reactant A is forming a product
- As the reactant will get converted into product, concentration of reactant will decrease and concentration of product will increase.
- Rate of reaction over here will depend upon change in concentration of A. Faster the concentration will decrease: higher will be the rate of reaction.
- Whenever in chemistry we talk, the average rate always remembers that the average rate will always be the change in physical quantity in a time interval which is not very small.

- here we are measuring the concentration of reactant A at two time ${{t}_{1}}$ and time ${{t}_{2}}$ respectively are ${{A}_{1}}$ and${{A}_{2}}$ where ${{A}_{2}}
$\dfrac{{{A}_{2}}-{{A}_{1}}}{{{t}_{2}}-{{t}_{1}}}$
- Now because concentration of A is decreasing with time and ${{A}_{2}}<{{A}_{1}}$ that’s why this expressing can be written as follow to express the average rate of reaction
$r=-\dfrac{\Delta A}{\Delta t}$
$\Delta A$ is change in concentration and $\Delta t$ is change in time.
- Now change in time is positive but with respect to that change in concentration is negative, so their ratio will be negative but rate of reaction can’t be negative that’s why a negative sign is applied before the expression to keep the rate of reaction positive.
So the significance of negative sign is to show the decrease in concentration of reactant.
- When the time interval $\Delta t$ becomes very small this average rate of reaction becomes an instantaneous rate of reaction.
$r={}_{\Delta t\to 0}\dfrac{-\Delta A}{\Delta t}$
\[r=-\dfrac{dA}{dt}\]
Expression for instantaneous rate of reaction is \[r=-\dfrac{dA}{dt}\]
Note: There is branch of physical chemistry known as chemical kinetics where we study about the rates of different reactions and classify them as fast, moderate and slow reactions based on their rate and we also study about the various factors like temperature, pressure etc. those affect the rate of reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




