
Match the following:
Natural Source Acid 1. Vinegar A. Oxalic Acid 2. Orange B. Acetic Acid 3. Tamarind C. Citric Acid 4. Tomato D. Tartaric acid
A. 1-a, 2-b, 3-d, 4-c
B. 1-b, 2-c, 3-d, 4-a
C. 1-a, 2-c, 3-d, 4-a
D. 1-d, 2-a, 3-b, 4-c
| Natural Source | Acid |
| 1. Vinegar | A. Oxalic Acid |
| 2. Orange | B. Acetic Acid |
| 3. Tamarind | C. Citric Acid |
| 4. Tomato | D. Tartaric acid |
Answer
569.7k+ views
Hint: The chemical formula and name of the acid present in the vinegar is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}$ ethanoic acid. The chemical formula and name of the acid present in the orange is ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{8}}}{{\text{O}}_7}$ $2 - $hydroxy propane$ - 1,2,3 - $tricarboxylic acid. The chemical formula and name of the acid present in the tamarind is ${{\text{C}}_4}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{6}}}$$2,3 - $Dihydroxy butanedioic acid. The chemical formula and name of the acid present in the tomato is ${{\text{C}}_2}{{\text{H}}_2}{{\text{O}}_4}$ ethanedioic acid.
Complete answer:
A molecule that can donate a proton or accept electrons is known as acid.
The acid present in vinegar is acetic acid which is systematically known as ethanoic acid. It is a weak acid. The chemical formula of the acetic acid is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}$.
The structure of acetic acid is as follows:
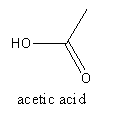
The acid present in orange is citric acid which is systematically known as $2 - $ hydroxy propane$ - 1,2,3 - $tricarboxylic acid. It is soluble in water. The molecular formula of the citric acid is ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{8}}}{{\text{O}}_7}$.
The structure of citric acid is as follows:
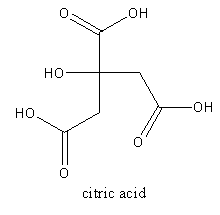
The acid present in tamarind is tartaric acid. It is an alpha-hydroxy carboxylic acid. The molecular formula of the tartaric acid is ${{\text{C}}_4}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{6}}}$. The IUPAC name of tartaric acid is $2,3 - $Dihydroxy butanedioic acid.
The structure of tartaric acid is as follows:
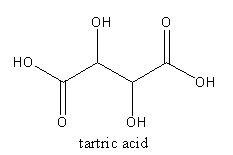
The acid present in tomato is oxalic acid. The molecular formula of the tartaric acid is ${{\text{C}}_2}{{\text{H}}_2}{{\text{O}}_4}$. The systematic name of oxalic acid is ethanedioic acid.
The structure of oxalic acid is as follows:
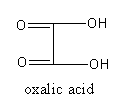
So, correct match of natural source and acid are as following:
Therefore, option (B) 1-b, 2-c, 3-d, 4-a, is correct.
Note: All acids have two carboxylic groups. The substance which has acids has a sour test. The pH of the liquid of these substances will be less than seven. Liquid of these substances can convert blue litmus into red which confirms the acidic nature of these substances. The main acid present in orange is ascorbic acid or vitamin C having molecular formula ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{6}}}$. Ascorbic acid or vitamin C cannot be produced by the body and cannot be stored. It is obtained from the diet. It is necessary for the recovery of the tissues. Fruit and vegetables are rich sources of ascorbic acid.
Complete answer:
A molecule that can donate a proton or accept electrons is known as acid.
The acid present in vinegar is acetic acid which is systematically known as ethanoic acid. It is a weak acid. The chemical formula of the acetic acid is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}$.
The structure of acetic acid is as follows:

The acid present in orange is citric acid which is systematically known as $2 - $ hydroxy propane$ - 1,2,3 - $tricarboxylic acid. It is soluble in water. The molecular formula of the citric acid is ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{8}}}{{\text{O}}_7}$.
The structure of citric acid is as follows:

The acid present in tamarind is tartaric acid. It is an alpha-hydroxy carboxylic acid. The molecular formula of the tartaric acid is ${{\text{C}}_4}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{6}}}$. The IUPAC name of tartaric acid is $2,3 - $Dihydroxy butanedioic acid.
The structure of tartaric acid is as follows:

The acid present in tomato is oxalic acid. The molecular formula of the tartaric acid is ${{\text{C}}_2}{{\text{H}}_2}{{\text{O}}_4}$. The systematic name of oxalic acid is ethanedioic acid.
The structure of oxalic acid is as follows:

So, correct match of natural source and acid are as following:
| Natural Source | Acid |
| 1. Vinegar | B. Acetic Acid |
| 2. Orange | C. Citric Acid |
| 3. Tamarind | D. Tartaric Acid |
| 4. Tomato | A. Oxalic Acid |
Therefore, option (B) 1-b, 2-c, 3-d, 4-a, is correct.
Note: All acids have two carboxylic groups. The substance which has acids has a sour test. The pH of the liquid of these substances will be less than seven. Liquid of these substances can convert blue litmus into red which confirms the acidic nature of these substances. The main acid present in orange is ascorbic acid or vitamin C having molecular formula ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{6}}}$. Ascorbic acid or vitamin C cannot be produced by the body and cannot be stored. It is obtained from the diet. It is necessary for the recovery of the tissues. Fruit and vegetables are rich sources of ascorbic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




