
Mass spectrum of lead is given as, the relative atomic mass of lead is?
A.\[208\]
B.\[207.567\]
C.\[207.302\]
D.\[209\]

Answer
525.9k+ views
Hint: A mass spectrum is defined as the distribution of ions by the use of mass spectrophotometer or mass spectrograph. Mass spectrometer is used to determine the molecular weight of an unknown sample.
Complete answer:
This method is very effective in the laboratories to find the unknown atomic weight.
To solve this question, we need to have basic knowledge about isotopes.
So, isotopes are the two or more types of the atom that have the same atomic number but different atomic weight.
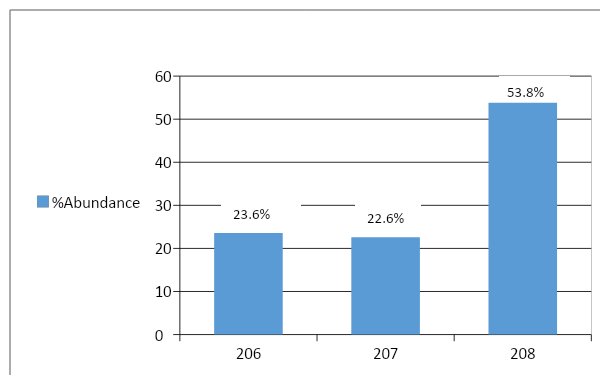
Here, in the diagram, we are given the mass spectrum of different isotopes of the lead.
\[Pb\] \[ - \] \[206\] Shows a peak absorption of mass spectrum at \[23.6\% \]
\[Pb\] \[ - \] \[207\] Shows a peak absorption of mass spectrum at \[22.6\% \]
\[Pb\] \[ - \] \[208\] Shows a peak absorption of mass spectrum at \[53.8\% \]
So, let’s calculate the atomic mass:
Formula to calculate the atomic mass using mass spectrum,
Atomic weight \[ = \dfrac{{(206 \times 23.3 + 207 \times 22.6 + 208 \times 53.8)}}{{100}}\% \]
\[ = 207.302\% \]
So, the atomic mass of lead, based on the given mass spectrum is \[ = 207.302\% \]
Note:
It is measured using an instrument known as a spectrophotometer. It is a sensitive and specific process. The main applications of mass spectrophotometry is in proteomics i.e. in the study of proteins about their structure, interactions with other molecules and their mass. Other than in proteins, the mass spectrometry is useful in microbiological fields like in drug identification and to find the contamination in the food items, pesticide residue analysis. Mass spectroscopy is also found useful in carbon dating.
Complete answer:
This method is very effective in the laboratories to find the unknown atomic weight.
To solve this question, we need to have basic knowledge about isotopes.
So, isotopes are the two or more types of the atom that have the same atomic number but different atomic weight.
Here, in the diagram, we are given the mass spectrum of different isotopes of the lead.
\[Pb\] \[ - \] \[206\] Shows a peak absorption of mass spectrum at \[23.6\% \]
\[Pb\] \[ - \] \[207\] Shows a peak absorption of mass spectrum at \[22.6\% \]
\[Pb\] \[ - \] \[208\] Shows a peak absorption of mass spectrum at \[53.8\% \]
So, let’s calculate the atomic mass:
Formula to calculate the atomic mass using mass spectrum,
Atomic weight \[ = \dfrac{{(206 \times 23.3 + 207 \times 22.6 + 208 \times 53.8)}}{{100}}\% \]
\[ = 207.302\% \]
So, the atomic mass of lead, based on the given mass spectrum is \[ = 207.302\% \]
Note:
It is measured using an instrument known as a spectrophotometer. It is a sensitive and specific process. The main applications of mass spectrophotometry is in proteomics i.e. in the study of proteins about their structure, interactions with other molecules and their mass. Other than in proteins, the mass spectrometry is useful in microbiological fields like in drug identification and to find the contamination in the food items, pesticide residue analysis. Mass spectroscopy is also found useful in carbon dating.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




