
Major product P will be:
\[Ph-C\equiv C-C{{H}_{3}}\xrightarrow{{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}}P\]
A.
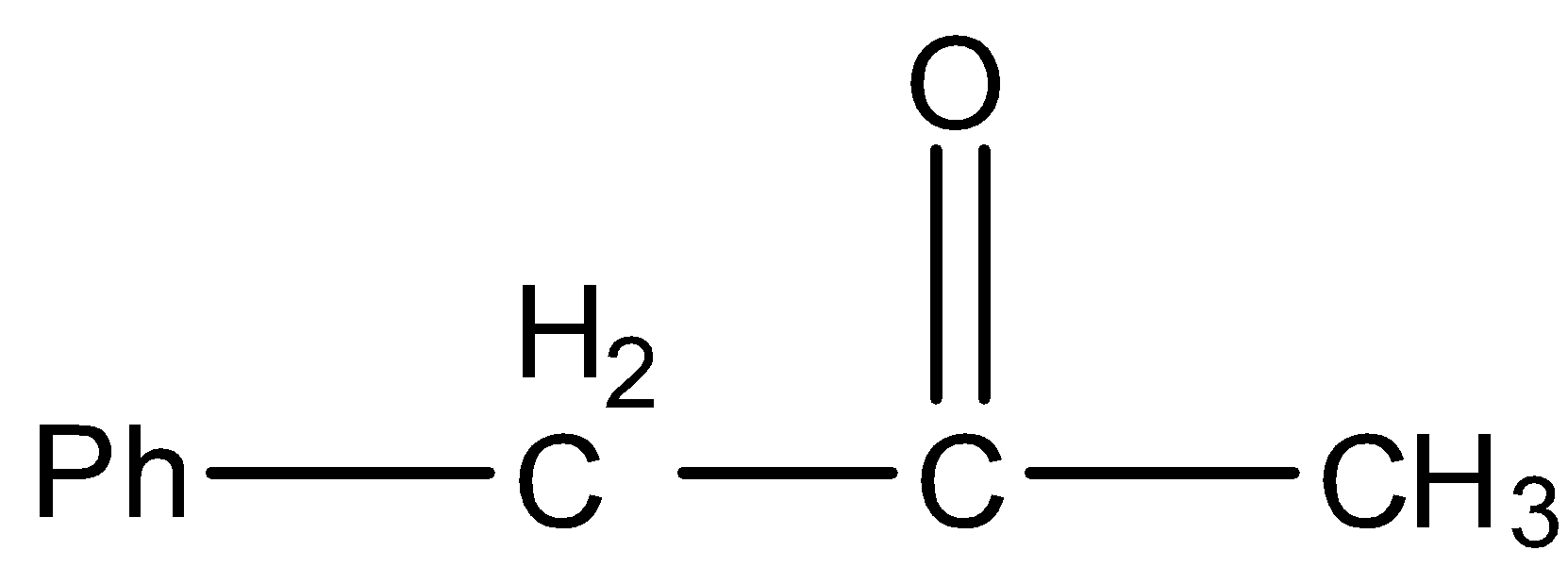
B.
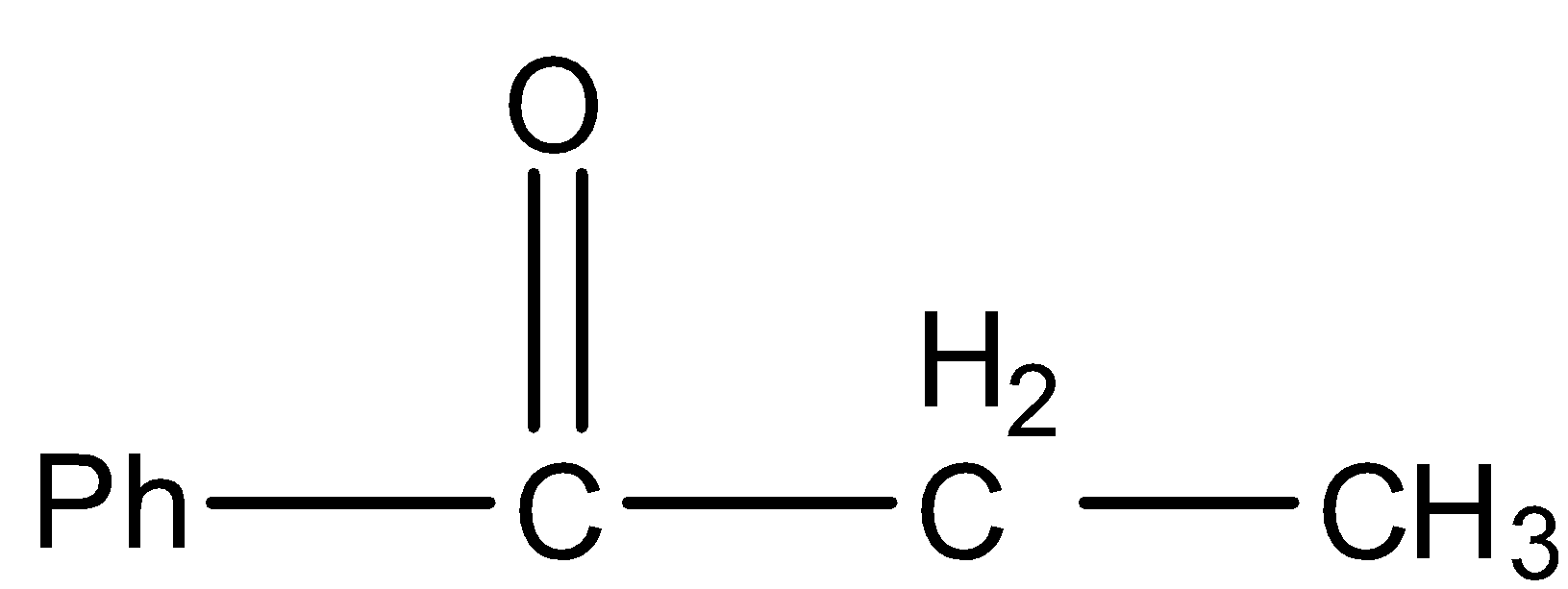
C.
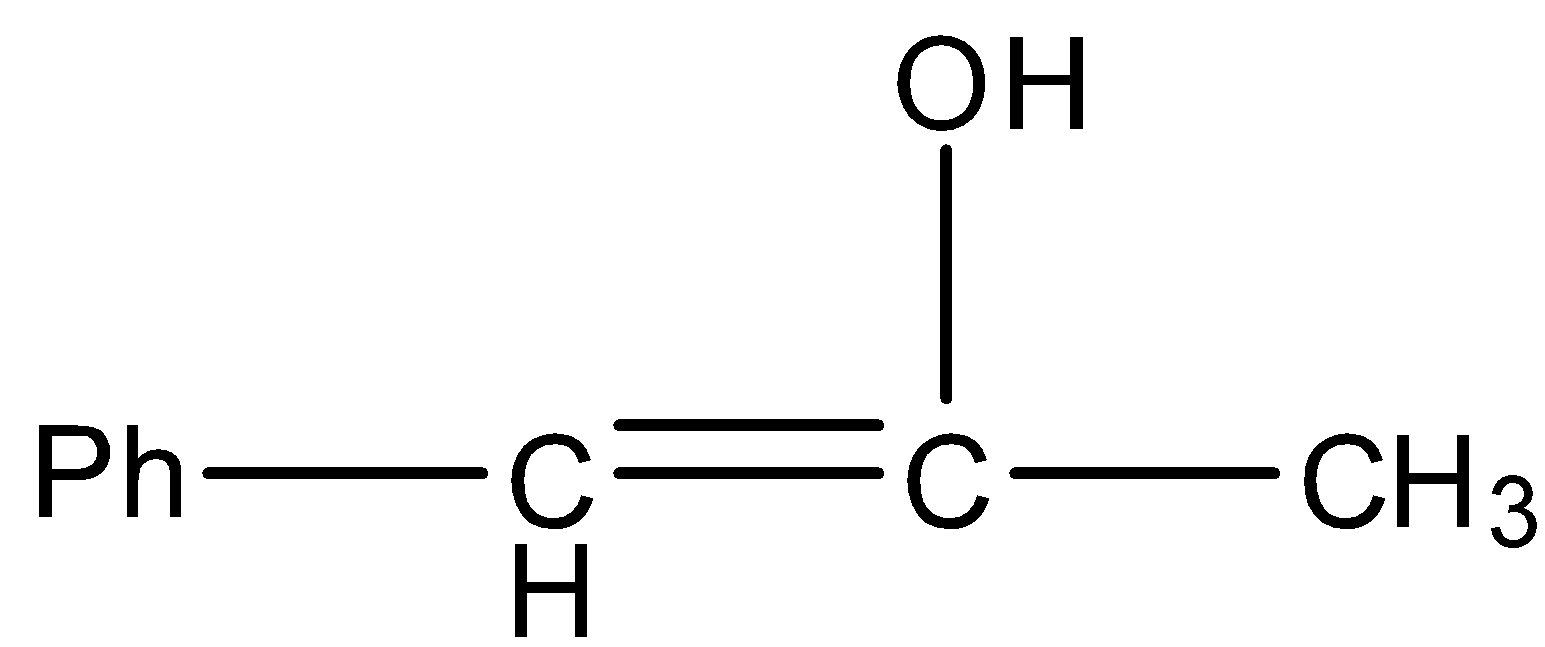
D.
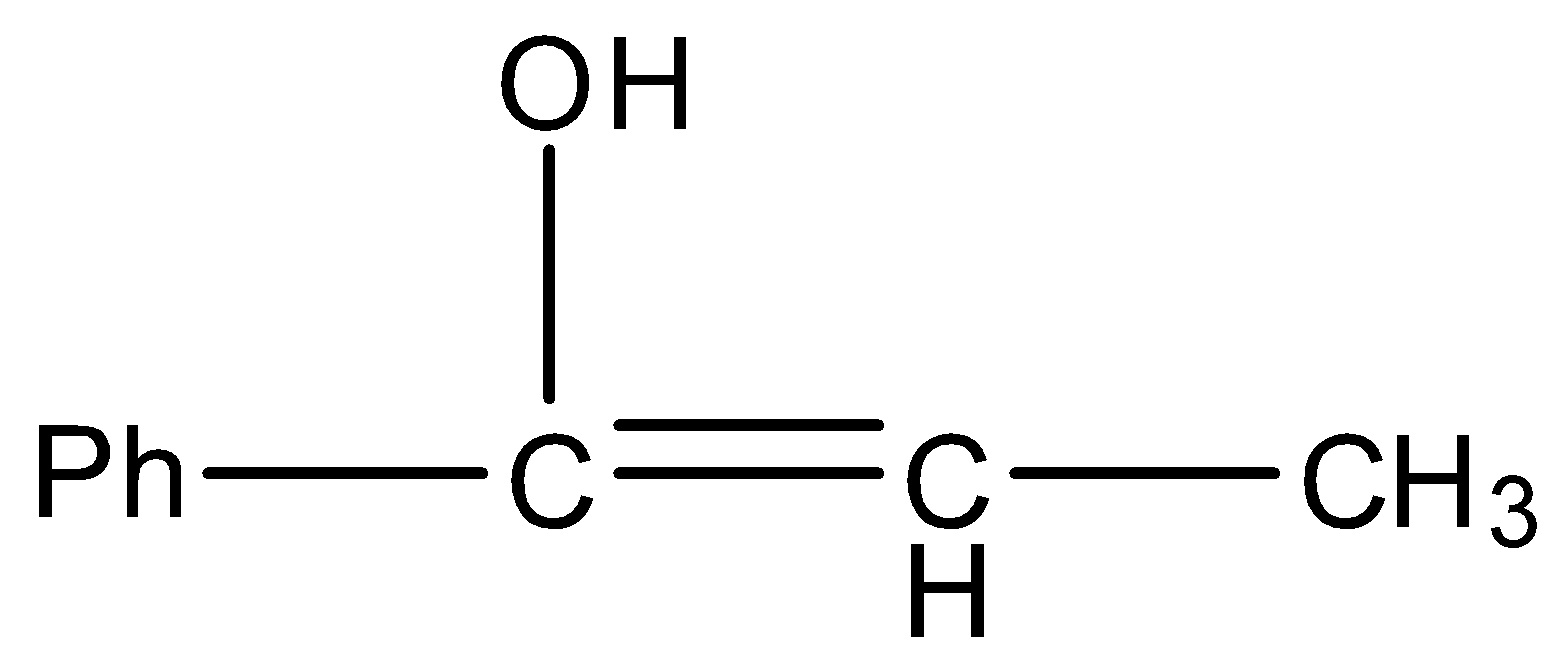




Answer
572.1k+ views
Hint: Generally alkynes undergo hydration reactions easily in the presence of concentrated sulphuric acid when they are attached to electron rich substituents. It is an example of oxidation reaction on alkenes by using sulphuric acid and water.
Complete Solution :
- In the question it is given that sulphuric acid is going to react with an alkyne and we have to find the product among the given options.
- The chemical reaction is as follows:
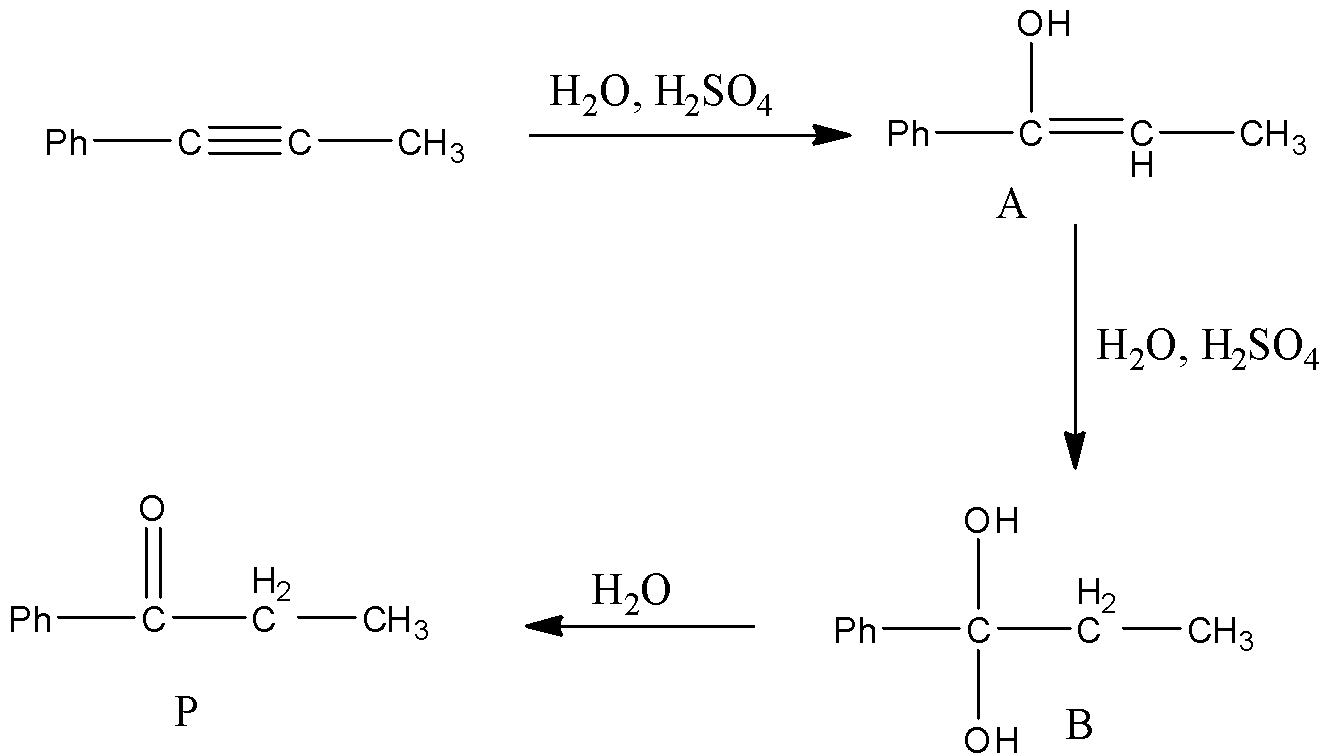
- In the above reaction at starting the hydroxyl group in water reacts with the carbon which is attached to the phenyl group and forms a hydroxyl alkene (A) as the product.
- The formed product A reacts with another mole of water in presence of an acid and forms diol derivative (B) as the product.
- The diol product (B) is unstable because of the presence of two hydroxyl groups on the same carbon.
- Then the product (B) undergoes dehydration reaction and forms a product (P) which contains a ketone group which is adjacent to the phenyl group.
- Therefore the structure of the formed product P is as follows.
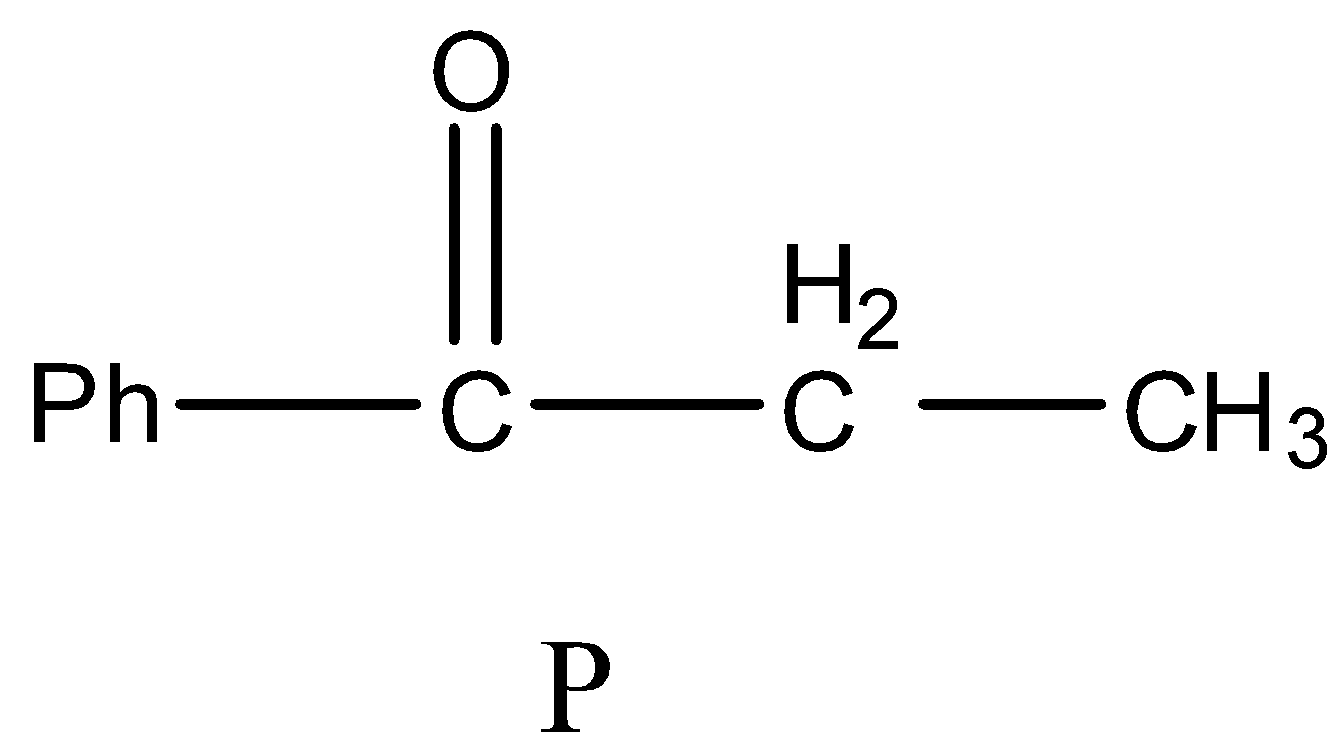
So, the correct answer is “Option B”.
Note: This reaction is an example of a hydration reaction. Because two hydrogens are added to one carbon and one oxygen atom is added to another carbon in the same molecule in presence of water and sulphuric acid. In the above reactions an unsaturated alkyne compound is converted to a ketone.
Complete Solution :
- In the question it is given that sulphuric acid is going to react with an alkyne and we have to find the product among the given options.
- The chemical reaction is as follows:

- In the above reaction at starting the hydroxyl group in water reacts with the carbon which is attached to the phenyl group and forms a hydroxyl alkene (A) as the product.
- The formed product A reacts with another mole of water in presence of an acid and forms diol derivative (B) as the product.
- The diol product (B) is unstable because of the presence of two hydroxyl groups on the same carbon.
- Then the product (B) undergoes dehydration reaction and forms a product (P) which contains a ketone group which is adjacent to the phenyl group.
- Therefore the structure of the formed product P is as follows.

So, the correct answer is “Option B”.
Note: This reaction is an example of a hydration reaction. Because two hydrogens are added to one carbon and one oxygen atom is added to another carbon in the same molecule in presence of water and sulphuric acid. In the above reactions an unsaturated alkyne compound is converted to a ketone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




