
How many lone pairs are present in $${N_2}{O_4}$$ ?
Answer
553.8k+ views
Hint: Lone pair is the non bonding pair or the unshared pair which does not take part in the bonding of covalent bond. It is the pair of valence electrons which is not shared with another atom. While bonds pair those which take part in the bonding and share its electron.
Complete step by step answer:
The Lewis structure of $${N_2}{O_4}$$is given below,
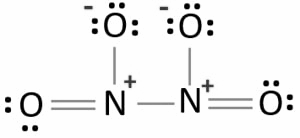
In the above structure we can see that one nitrogen atom is bonded to another nitrogen atom by a single bond. Both of the nitrogen atoms form N-O bonds. Two of the four N-O bonds are single bonded and other two are double bonded. The total number of valence electrons by nitrogen atoms are 10. The valence electrons given by four oxygen atoms are 24. So the total valence electron will be 34. The total valence electron pair will be 17. After bonding we can see in the structure that 10 pairs of electrons are not able to take part in the bonding so they are unshared. These remaining 10 pairs of valence electrons are called lone pairs. The Lewis structure is said to be stable when the charges on the atom are least. But in the above structure all oxygen atoms have one negative charge and nitrogen atoms have two positive charges. So it is not the most stable structure of $${N_2}{O_4}$$.The structure with the stable one with less charge is the Lewis structure below. The oxygen atom in this structure has one negative charge each except the double bonded oxygen and the one nitrogen atom attached to double bonded oxygen has one positive charge and the other has two positive charges.
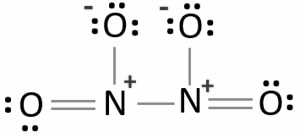
Note: Lewis structure is the dot diagram of the molecule. It is used to represent the valence electrons of the atom of the molecule. To write the Lewis structure you should always find the total number of valence electrons, then determine the bonded pairs of electrons . After this just subtract the two then you can get the lone pairs of the molecule. Then just place the electrons around the skeletal structure of the molecule.
Complete step by step answer:
The Lewis structure of $${N_2}{O_4}$$is given below,
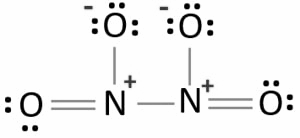
In the above structure we can see that one nitrogen atom is bonded to another nitrogen atom by a single bond. Both of the nitrogen atoms form N-O bonds. Two of the four N-O bonds are single bonded and other two are double bonded. The total number of valence electrons by nitrogen atoms are 10. The valence electrons given by four oxygen atoms are 24. So the total valence electron will be 34. The total valence electron pair will be 17. After bonding we can see in the structure that 10 pairs of electrons are not able to take part in the bonding so they are unshared. These remaining 10 pairs of valence electrons are called lone pairs. The Lewis structure is said to be stable when the charges on the atom are least. But in the above structure all oxygen atoms have one negative charge and nitrogen atoms have two positive charges. So it is not the most stable structure of $${N_2}{O_4}$$.The structure with the stable one with less charge is the Lewis structure below. The oxygen atom in this structure has one negative charge each except the double bonded oxygen and the one nitrogen atom attached to double bonded oxygen has one positive charge and the other has two positive charges.
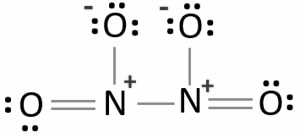
Note: Lewis structure is the dot diagram of the molecule. It is used to represent the valence electrons of the atom of the molecule. To write the Lewis structure you should always find the total number of valence electrons, then determine the bonded pairs of electrons . After this just subtract the two then you can get the lone pairs of the molecule. Then just place the electrons around the skeletal structure of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




