
IUPAC name of the formula $C{{H}_{2}}{{(COOH)}_{2}}$ is?
A. Butanoic acid
B. $\text{Propane}-1,3-\text{dioic acid}$
C. $\text{Propane}-1,2-\text{dioic acid}$
D. Malonic acid
Answer
565.8k+ views
Hint: We should know that IUPAC name is determined by the number of carbon atoms present in the hydrocarbon chain. So, first count the atoms of carbon in the given formula and give the name according to the number of carbons. Then define the IUPAC name by what type of group is attached in the branch of the chain.
Complete answer:
IUPAC name is defined by the counting number of carbon atoms. There are different names given by the numbers of carbon atoms. If the molecule contains one carbon, then it is named as methen, if there are two carbons it is known as ethen.
So, here we can see it has three carbon atoms in the given formula $C{{H}_{2}}{{(COOH)}_{2}}$. So, it will be known by the name propane (as it has single covalent bonds attached to the side groups with the carbon atoms). And we can see there are two carboxylic acids. So, the suffix will be known by dioic. The carboxylic acids are present in the first and third carbon atom. So, the IUPAC name will be $\text{Propane}-1,3-\text{dioic acid}$ and its structure is shown as below,
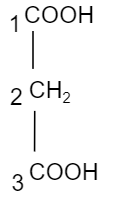
Hence, the correct option is B.
Note:
The possible mistake could be you can get confused with option D i.e. malonic acid. The given formula with IUPAC name $\text{Propane}-1,3-\text{dioic acid}$ is also known by the common name “malonic acid”. Remember, the common name and IUPAC nomenclature are two different entities. IUPAC nomenclature is a method which depends on the chain of carbon atoms and the group attached to it.
Complete answer:
IUPAC name is defined by the counting number of carbon atoms. There are different names given by the numbers of carbon atoms. If the molecule contains one carbon, then it is named as methen, if there are two carbons it is known as ethen.
So, here we can see it has three carbon atoms in the given formula $C{{H}_{2}}{{(COOH)}_{2}}$. So, it will be known by the name propane (as it has single covalent bonds attached to the side groups with the carbon atoms). And we can see there are two carboxylic acids. So, the suffix will be known by dioic. The carboxylic acids are present in the first and third carbon atom. So, the IUPAC name will be $\text{Propane}-1,3-\text{dioic acid}$ and its structure is shown as below,

Hence, the correct option is B.
Note:
The possible mistake could be you can get confused with option D i.e. malonic acid. The given formula with IUPAC name $\text{Propane}-1,3-\text{dioic acid}$ is also known by the common name “malonic acid”. Remember, the common name and IUPAC nomenclature are two different entities. IUPAC nomenclature is a method which depends on the chain of carbon atoms and the group attached to it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




