
IUPAC name for the following structure:
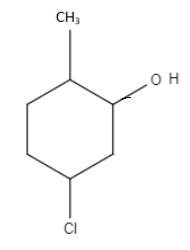
A.2-methyl-5chlorocyclohexonol
B.2-chloro-4-methylcyclohexanol
C.5-chloro-2-methylcyclohexanol
D.3-chloro-2-methylcyclohexanol

Answer
552.6k+ views
Hint: In synthetic terminology, the IUPAC classification of natural science is a technique for naming natural substance mixes as suggested by the International Union of Pure and Applied Chemistry (IUPAC). It is distributed in the Nomenclature of Organic Chemistry.
Complete step by step answer:
When naming subbed cycloalkanes the numbering of the carbons is significant. When there is a solitary liquor bunch on a cycloalkane it is given the number 1 position and the cycloalkane is a cycloalkane. The quantity of the carbon the liquor is on is gathered through the show. The other substituent positions are then numbered regarding the liquor with the goal that they have the most minimal potential numbers. Classification is characterized as an arrangement of names and terms utilized in a specific field of study or network. An illustration of classification is the language of the model. An arrangement of names utilized in workmanship or science. The classification of mineralogy.
Cyclohexane is the organic compound with the formula \[HOCH{(C{H_2})_5}\]. The molecule is related to the cyclohexane ring by replacement of one hydrogen atom by a hydroxyl group. This compound exists as a deliquescent colourless solid with a camphor-like odor, which, when very pure, melts near room temperature. Cyclohexanol is liquor. Liquor contains an OH bunch associated with a tetrahedral carbon. Phenol is marginally unique; it contains an OH bunch associated with a three-sided planar carbon that is important for a sweet-smelling ring. Cyclohexanol has a pKa of around 18. Hence the correct answer is 5-chloro-2-methylcyclohexanol, option (C).
So, the correct answer is Option C.
Note: To name natural mixes, you should initially retain a couple of essential names. These names are recorded inside the conversation of naming alkanes. By and large, the base piece of the name mirrors the quantity of carbons in what you have appointed to be the parent chain. The postfix of the name mirrors the type(s) of the practical group(s) present on (or inside) the parent chain. Different gatherings that are joined to the parent chain are called substituents.
Complete step by step answer:
When naming subbed cycloalkanes the numbering of the carbons is significant. When there is a solitary liquor bunch on a cycloalkane it is given the number 1 position and the cycloalkane is a cycloalkane. The quantity of the carbon the liquor is on is gathered through the show. The other substituent positions are then numbered regarding the liquor with the goal that they have the most minimal potential numbers. Classification is characterized as an arrangement of names and terms utilized in a specific field of study or network. An illustration of classification is the language of the model. An arrangement of names utilized in workmanship or science. The classification of mineralogy.
Cyclohexane is the organic compound with the formula \[HOCH{(C{H_2})_5}\]. The molecule is related to the cyclohexane ring by replacement of one hydrogen atom by a hydroxyl group. This compound exists as a deliquescent colourless solid with a camphor-like odor, which, when very pure, melts near room temperature. Cyclohexanol is liquor. Liquor contains an OH bunch associated with a tetrahedral carbon. Phenol is marginally unique; it contains an OH bunch associated with a three-sided planar carbon that is important for a sweet-smelling ring. Cyclohexanol has a pKa of around 18. Hence the correct answer is 5-chloro-2-methylcyclohexanol, option (C).
So, the correct answer is Option C.
Note: To name natural mixes, you should initially retain a couple of essential names. These names are recorded inside the conversation of naming alkanes. By and large, the base piece of the name mirrors the quantity of carbons in what you have appointed to be the parent chain. The postfix of the name mirrors the type(s) of the practical group(s) present on (or inside) the parent chain. Different gatherings that are joined to the parent chain are called substituents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




