
Is this possible? If yes, then how?
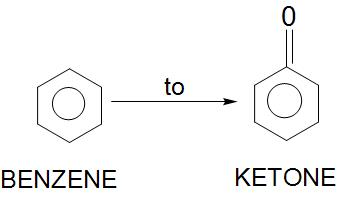

Answer
569.7k+ views
Hint: Benzene can be converted into ketone by electrophilic substitution reaction. During this reaction generation of an electrophile occurs. And the transformation of the aromatic ring into a ketone takes place.
Complete Solution :
- Benzene can be converted into ketone by Friedal craft acylation reaction.
- It is found that in Friedel-Crafts acylation reaction, the addition of an acyl group to an aromatic ring takes place. This reaction happens in presence of a Lewis acid catalyst like AlCl3.
- We can also use acid anhydride as an alternative to the acyl halide in Friedel-Crafts acylation reaction.
- Let’s discuss about the reaction that takes place between benzene and an acyl chloride:
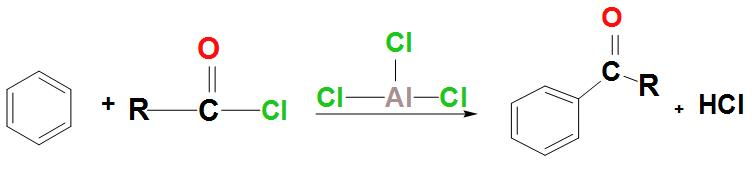
- Let’s discuss about the mechanism of Friedel-Crafts acylations
Friedel-Crafts acylations take place through a four-step mechanism.
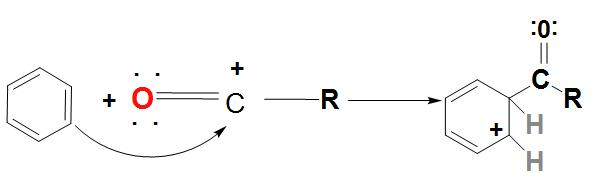
- In the first step, we can see that Lewis acid catalyst ($AlC{{l}_{3}}$) and the acyl halide react with each other and an acylium ion is formed, which is stabilized by resonance.
- In the next step the acylium ion ($RC{{O}^{+}}$) an electrophilic attack on the aromatic ring. As there is a complex formed, the aromaticity of the ring is found to be temporarily lost.
- It is found that the intermediate complex is deprotonated. The proton attaches to a chloride ion HCl and leads to regeneration of $AlC{{l}_{3}}$ catalyst.
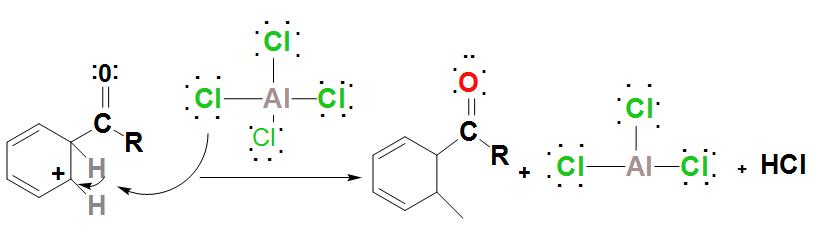
- Further, the regenerated catalyst is now found to attack the carbonyl oxygen. Hence, by adding water to the products that are formed in the above steps, the ketone product must be liberated.
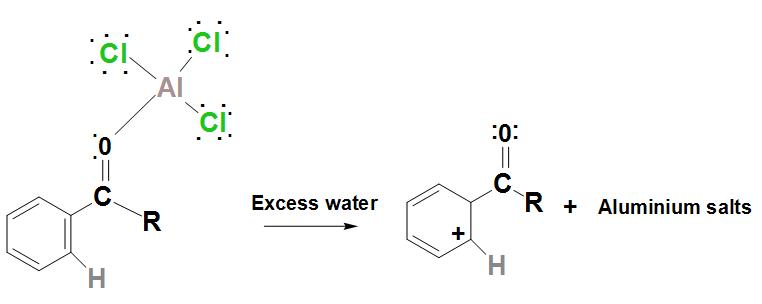
Hence, we can conclude that benzene can be converted into ketone by Friedel-Crafts acylation reaction.
Note: - Friedel-Crafts acylation has advantages over that of Friedel-Crafts alkylation. In Friedel-Crafts acylation, polyacrylate doesn’t take place as the product ketone is less reactive than that of reactant.
- Whereas, in case of Friedel-Crafts alkylation, polyalkylation takes place.
Complete Solution :
- Benzene can be converted into ketone by Friedal craft acylation reaction.
- It is found that in Friedel-Crafts acylation reaction, the addition of an acyl group to an aromatic ring takes place. This reaction happens in presence of a Lewis acid catalyst like AlCl3.
- We can also use acid anhydride as an alternative to the acyl halide in Friedel-Crafts acylation reaction.
- Let’s discuss about the reaction that takes place between benzene and an acyl chloride:

- Let’s discuss about the mechanism of Friedel-Crafts acylations
Friedel-Crafts acylations take place through a four-step mechanism.
- In the first step, we can see that Lewis acid catalyst ($AlC{{l}_{3}}$) and the acyl halide react with each other and an acylium ion is formed, which is stabilized by resonance.
- In the next step the acylium ion ($RC{{O}^{+}}$) an electrophilic attack on the aromatic ring. As there is a complex formed, the aromaticity of the ring is found to be temporarily lost.

- It is found that the intermediate complex is deprotonated. The proton attaches to a chloride ion HCl and leads to regeneration of $AlC{{l}_{3}}$ catalyst.

- Further, the regenerated catalyst is now found to attack the carbonyl oxygen. Hence, by adding water to the products that are formed in the above steps, the ketone product must be liberated.

Hence, we can conclude that benzene can be converted into ketone by Friedel-Crafts acylation reaction.
Note: - Friedel-Crafts acylation has advantages over that of Friedel-Crafts alkylation. In Friedel-Crafts acylation, polyacrylate doesn’t take place as the product ketone is less reactive than that of reactant.
- Whereas, in case of Friedel-Crafts alkylation, polyalkylation takes place.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




