
Increasing order of acidic strength of given compounds is:
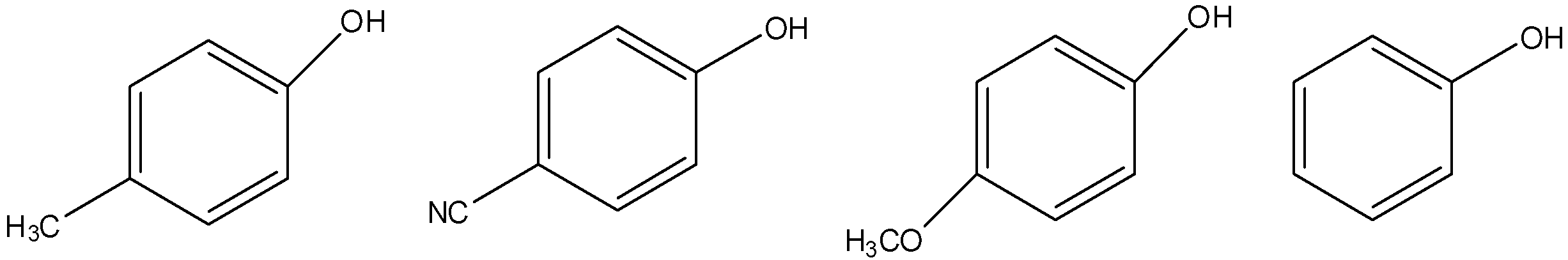
A.$III < I < IV < II$
B.$III < I < IV = II$
C.$III > I > IV > II$
D.$III < II < IV < I$

Answer
519.3k+ views
Hint: We have to know that the compound is less acidic if there is an electron withdrawing substituent bonded to the benzene ring. We have to know that compound is more acidic, when the benzene ring is bonded to an electron donating substituent.
Complete answer:
We are provided with four compounds that have phenol groups with several functional groups bonded to them. We know that a chemical species which donates hydrogen ions is an acid. We have to know that conjugate base is obtained when hydrogen ion is taken out from an acid. Strong acid could have a stable conjugate base. The charge on the conjugate base could be negative when an acid gives out hydrogen ion.
When the electron donating group is bonded, the density of electrons on the conjugate base increases. Because of this, the conjugate base turns more stable and compounds turn to be more acidic.
When the electron withdrawing group is bonded, the density of electrons on the conjugate base decreases. Because of this, the conjugate base turns less stable and compounds turn to be less acidic. The strength of acid is increased by the electron donating group whereas the strength of acid is increased by the electron withdrawing group.
In compound (1), we can see the presence of an electron donating methyl group. So, compound (1) is more acidic.
In compound (2), we can see the presence of an electron withdrawing cyano group. So, compound (2) is less acidic.
In compound (3), we can see the presence of an electron donating methoxy group. So, compound (3) is more acidic.
We have to know the methoxy group is more electron donating group when compared to methyl group. So, the acidity of compound (3) is more when compared to acidity of compound (1).
In compound (4), neither electron withdrawing group (or) electron donating group is found. So, compound (4) is more acidic when compared to compound (2) but less acidic when compared to compound (1) and compound (3).
So, we can write the increasing order of acidic strength as $III > I > IV > II$.
Option (C) is correct.
Note:
We have to remember that when we are deciding the acidic strength, we have to predict the stability of the conjugate base. We have to know that the compound would be less acidic, when the group that is electron withdrawing bonded to the benzene ring. We have to know that compound would be more acidic, the group is electron donating bonded to the benzene ring.
Complete answer:
We are provided with four compounds that have phenol groups with several functional groups bonded to them. We know that a chemical species which donates hydrogen ions is an acid. We have to know that conjugate base is obtained when hydrogen ion is taken out from an acid. Strong acid could have a stable conjugate base. The charge on the conjugate base could be negative when an acid gives out hydrogen ion.
When the electron donating group is bonded, the density of electrons on the conjugate base increases. Because of this, the conjugate base turns more stable and compounds turn to be more acidic.
When the electron withdrawing group is bonded, the density of electrons on the conjugate base decreases. Because of this, the conjugate base turns less stable and compounds turn to be less acidic. The strength of acid is increased by the electron donating group whereas the strength of acid is increased by the electron withdrawing group.
In compound (1), we can see the presence of an electron donating methyl group. So, compound (1) is more acidic.
In compound (2), we can see the presence of an electron withdrawing cyano group. So, compound (2) is less acidic.
In compound (3), we can see the presence of an electron donating methoxy group. So, compound (3) is more acidic.
We have to know the methoxy group is more electron donating group when compared to methyl group. So, the acidity of compound (3) is more when compared to acidity of compound (1).
In compound (4), neither electron withdrawing group (or) electron donating group is found. So, compound (4) is more acidic when compared to compound (2) but less acidic when compared to compound (1) and compound (3).
So, we can write the increasing order of acidic strength as $III > I > IV > II$.
Option (C) is correct.
Note:
We have to remember that when we are deciding the acidic strength, we have to predict the stability of the conjugate base. We have to know that the compound would be less acidic, when the group that is electron withdrawing bonded to the benzene ring. We have to know that compound would be more acidic, the group is electron donating bonded to the benzene ring.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




