
In which of the following species the angle around the central atom is exactly equal to ${{109}^{\circ }}{{28}^{'}}$
(A) $S{{F}_{4}}$
(B) $N{{H}_{3}}$
(C) $N{{H}_{4}}^{+}$
(D) None of these
Answer
584.4k+ views
Hint: To solve this, find the hybridisation of each of the given molecules. ${{109}^{\circ }}{{28}^{'}}$ is shown by a regular tetrahedral species. When the central metal atom is occupied in 4 bond pairs and no lone pairs, it will show a bond angle of ${{109}^{\circ }}{{28}^{'}}$.
Complete step by step answer:
We know that in chemistry, the angle between two bonds originating from the same atom in a covalent species is known as the bond angle.
Now let us discuss the relation of hybridisation with the bond angle.
Bond angle ${{109}^{\circ }}{{28}^{'}}$ is seen in species with regular tetrahedral geometry and they have a hybridisation of $s{{p}^{3}}$. For this hybridisation, steric number is 4. Steric number is the number of atoms, groups or lone pairs around the central metal atom.
$s{{p}^{3}}$ hybridized molecules may have different shapes and we can decide it by the steric number. For steric number 4, there are 4 structural possibilities but only one includes a bond angle of ${{109}^{\circ }}{{28}^{'}}$.
For bond angle to be ${{109}^{\circ }}{{28}^{'}}$, there must be 4 bond pairs and no lone pairs.
So now let’s check the molecules given to us and try to find out the number of bond pairs in them.
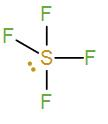
Firstly we have $S{{F}_{4}}$. It has a hybridisation of $s{{p}^{3}}d$ (Sulphur has 5 valence electrons. Four will be occupied by the 4 fluorine atoms and the other one will exist as a lone pair.) It will exist as a triangular bipyramidal and the bond angle is not ${{109}^{\circ }}{{28}^{'}}$ in it.
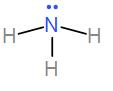
Then we have $N{{H}_{3}}$. Ammonia has three bond pairs and one lone pair and the bond angle is ${{107}^{\circ }}$.
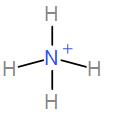
Lastly, we have $N{{H}_{4}}^{+}$. It has 4 bond pairs and no lone pair and it takes up tetrahedral geometry with $s{{p}^{3}}$ hybridisation. Therefore, the bond angle here is ${{109}^{\circ }}{{28}^{'}}$.
We can see from the above discussion that $N{{H}_{4}}^{+}$ has bond angle of ${{109}^{\circ }}{{28}^{'}}$ and this is the required answer.
Therefore, the correct answer is option (C) $N{{H}_{4}}^{+}$.
Note: $s{{p}^{3}}$hybridised molecules can have different shapes and it is decided by its steric number. This hybridisation has a steric number of 4. There are 4 possible structures and they are:
- Central atom occupied in 4 bond pairs will give a bond angle of ${{109}^{\circ }}{{28}^{'}}$ .
- Central atoms occupied in 3 bond pairs and 1 lone pair will show a bond angle of ${{107}^{\circ }}$.
- Central atoms occupied in 2 bond pairs and 2 lone pairs will show a bond angle of ${{104}^{\circ }}{{5}^{'}}$ .
- Central atoms occupied in 1 bond pair and 3 lone pairs will not have any defined bond angle as there is only one bond.
Complete step by step answer:
We know that in chemistry, the angle between two bonds originating from the same atom in a covalent species is known as the bond angle.
Now let us discuss the relation of hybridisation with the bond angle.
Bond angle ${{109}^{\circ }}{{28}^{'}}$ is seen in species with regular tetrahedral geometry and they have a hybridisation of $s{{p}^{3}}$. For this hybridisation, steric number is 4. Steric number is the number of atoms, groups or lone pairs around the central metal atom.
$s{{p}^{3}}$ hybridized molecules may have different shapes and we can decide it by the steric number. For steric number 4, there are 4 structural possibilities but only one includes a bond angle of ${{109}^{\circ }}{{28}^{'}}$.
For bond angle to be ${{109}^{\circ }}{{28}^{'}}$, there must be 4 bond pairs and no lone pairs.
So now let’s check the molecules given to us and try to find out the number of bond pairs in them.
Firstly we have $S{{F}_{4}}$. It has a hybridisation of $s{{p}^{3}}d$ (Sulphur has 5 valence electrons. Four will be occupied by the 4 fluorine atoms and the other one will exist as a lone pair.) It will exist as a triangular bipyramidal and the bond angle is not ${{109}^{\circ }}{{28}^{'}}$ in it.

Then we have $N{{H}_{3}}$. Ammonia has three bond pairs and one lone pair and the bond angle is ${{107}^{\circ }}$.

Lastly, we have $N{{H}_{4}}^{+}$. It has 4 bond pairs and no lone pair and it takes up tetrahedral geometry with $s{{p}^{3}}$ hybridisation. Therefore, the bond angle here is ${{109}^{\circ }}{{28}^{'}}$.

We can see from the above discussion that $N{{H}_{4}}^{+}$ has bond angle of ${{109}^{\circ }}{{28}^{'}}$ and this is the required answer.
Therefore, the correct answer is option (C) $N{{H}_{4}}^{+}$.
Note: $s{{p}^{3}}$hybridised molecules can have different shapes and it is decided by its steric number. This hybridisation has a steric number of 4. There are 4 possible structures and they are:
- Central atom occupied in 4 bond pairs will give a bond angle of ${{109}^{\circ }}{{28}^{'}}$ .
- Central atoms occupied in 3 bond pairs and 1 lone pair will show a bond angle of ${{107}^{\circ }}$.
- Central atoms occupied in 2 bond pairs and 2 lone pairs will show a bond angle of ${{104}^{\circ }}{{5}^{'}}$ .
- Central atoms occupied in 1 bond pair and 3 lone pairs will not have any defined bond angle as there is only one bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




