
In which of the following pairs of carbanions, the first one is more stable than the second?
A.\[\mathop C\limits^ - {F_3},\mathop C\limits^ - C{l_3}\]
B.\[HC = \mathop C\limits^ - ,{H_2}C = \mathop C\limits^ - H\]
C.
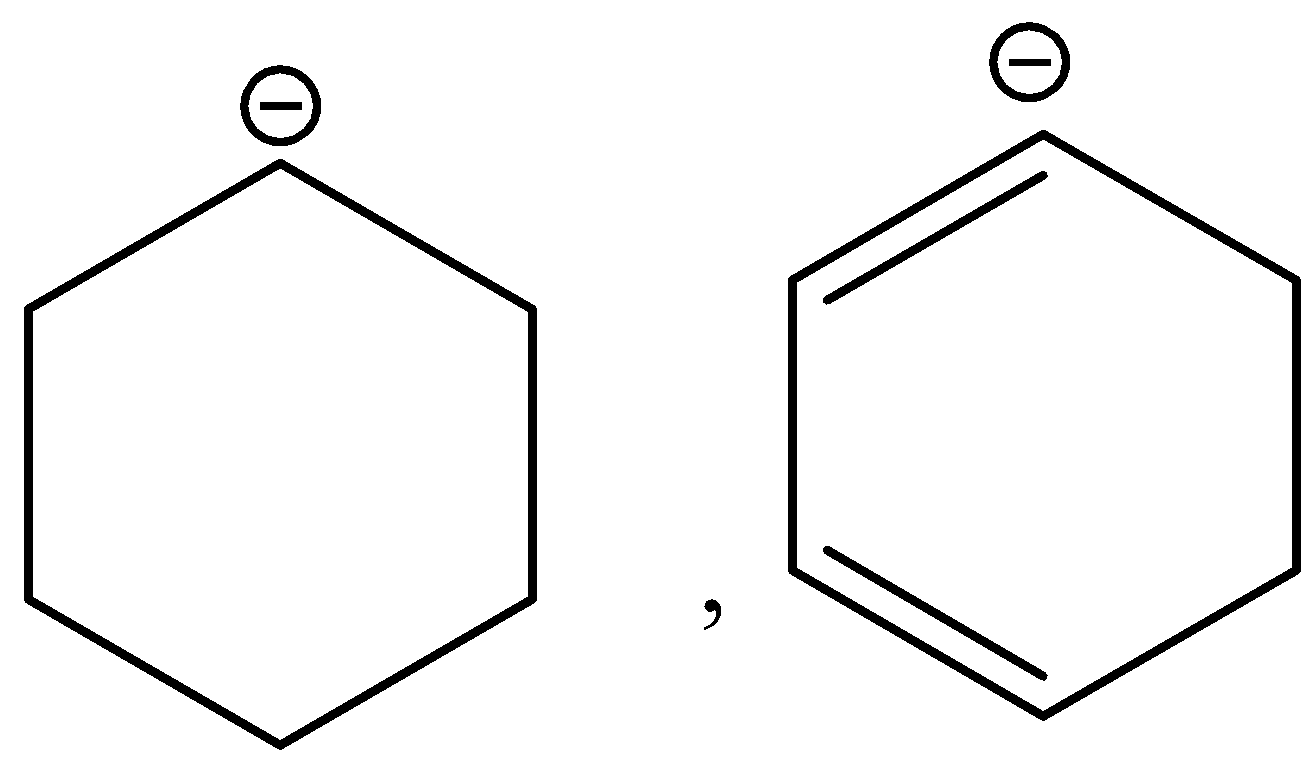
D.\[{\left( {C{H_3}} \right)_3}\mathop C\limits^ - ,{H_3}C - \mathop C\limits^ - {H_3}\]

Answer
544.8k+ views
Hint: A carbanion refers to an anion in which carbon possesses a formal negative charge and carbon is trivalent. There is an unshared pair of electrons. The stability of carbanion depends on so many factors like hybridization, electronegativity, resonance, and many more.
Complete step by step answer:
First, we will understand the basic representation of carbanion. The carbanions are represented as \[{R_3}C{:^ - }\]. According to the question, we need to find the pairs of carbanion in which the first one is more stable than the second. So, we will discuss all the pairs one by one and observe which pair of carbanions the first one is more stable as compared to the second.
The first pair is \[\mathop C\limits^ - {F_3},\mathop C\limits^ - C{l_3}\]. We know that \[\mathop C\limits^ - {F_3}\] fluorine decreases the electron density on carbon atoms as compared to chlorine \[\mathop C\limits^ - C{l_3}\]. So, the first carbanion is more stable as compared to the second.
In option (B) \[HC = \mathop C\limits^ - ,{H_2}C = \mathop C\limits^ - H\]. The second carbanion is more stable as compared to the first due to the greater number of hydrogen atoms attached to the carbanion.
Now when we will observe option (C). Here, we know that stability of the carbanion increases due to resonance. Therefore, the second carbanion is more stable as compared to the first one.
At last, we will compare the pair \[{\left( {C{H_3}} \right)_3}\mathop C\limits^ - ,{H_3}C - \mathop C\limits^ - {H_3}\]. In this pair also the second carbanion is more stable as compared to the first one. As the second carbanion is primary and the first carbanion is tertiary.
Therefore, the correct option is (A).
Note:
In the first pair, \[\mathop C\limits^ - {F_3},\mathop C\limits^ - C{l_3}\] the fluorine is more electronegative as compared to chlorine and the electron-withdrawing capacity of fluorine is also greater as compared to chlorine. Therefore, the first carbanion is more stable than the second. Carbanion also acts as a nucleophile.
Complete step by step answer:
First, we will understand the basic representation of carbanion. The carbanions are represented as \[{R_3}C{:^ - }\]. According to the question, we need to find the pairs of carbanion in which the first one is more stable than the second. So, we will discuss all the pairs one by one and observe which pair of carbanions the first one is more stable as compared to the second.
The first pair is \[\mathop C\limits^ - {F_3},\mathop C\limits^ - C{l_3}\]. We know that \[\mathop C\limits^ - {F_3}\] fluorine decreases the electron density on carbon atoms as compared to chlorine \[\mathop C\limits^ - C{l_3}\]. So, the first carbanion is more stable as compared to the second.
In option (B) \[HC = \mathop C\limits^ - ,{H_2}C = \mathop C\limits^ - H\]. The second carbanion is more stable as compared to the first due to the greater number of hydrogen atoms attached to the carbanion.
Now when we will observe option (C). Here, we know that stability of the carbanion increases due to resonance. Therefore, the second carbanion is more stable as compared to the first one.
At last, we will compare the pair \[{\left( {C{H_3}} \right)_3}\mathop C\limits^ - ,{H_3}C - \mathop C\limits^ - {H_3}\]. In this pair also the second carbanion is more stable as compared to the first one. As the second carbanion is primary and the first carbanion is tertiary.
Therefore, the correct option is (A).
Note:
In the first pair, \[\mathop C\limits^ - {F_3},\mathop C\limits^ - C{l_3}\] the fluorine is more electronegative as compared to chlorine and the electron-withdrawing capacity of fluorine is also greater as compared to chlorine. Therefore, the first carbanion is more stable than the second. Carbanion also acts as a nucleophile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




