
In which of the following option(s) all species contain $X-O-X$ bond(s) in structure (X= central atom)? (This question has multiple correct options).
(a)- ${{H}_{2}}{{S}_{2}}{{O}_{5}},\text{ }{{S}_{3}}{{O}_{9}},\text{ }{{S}_{2}}O_{6}^{2-}$
(b)- ${{P}_{4}}{{O}_{10}},\text{ }{{P}_{4}}{{O}_{6}},\text{ }{{H}_{5}}{{P}_{3}}{{O}_{10}}$
(c)- ${{N}_{2}}{{O}_{5}},\,\text{ }{{N}_{2}}O,\text{ }{{N}_{2}}{{O}_{4}}$
(d)- ${{S}_{3}}{{O}_{9}},\text{ }{{P}_{4}}{{O}_{6}},\ S{{i}_{2}}O_{7}^{6-}$
Answer
583.2k+ views
Hint: All the compounds given in the above option are oxoacids of sulfur, phosphorus, nitrogen, and silicon. Since oxoacids are made up of many elements, $X-O-X$ bonds are formed easily.
Complete step by step answer:
All the compounds given in the above options are oxoacids of sulfur, phosphorus, nitrogen, and silicon. Let us see the structure of all the compounds in the given options.
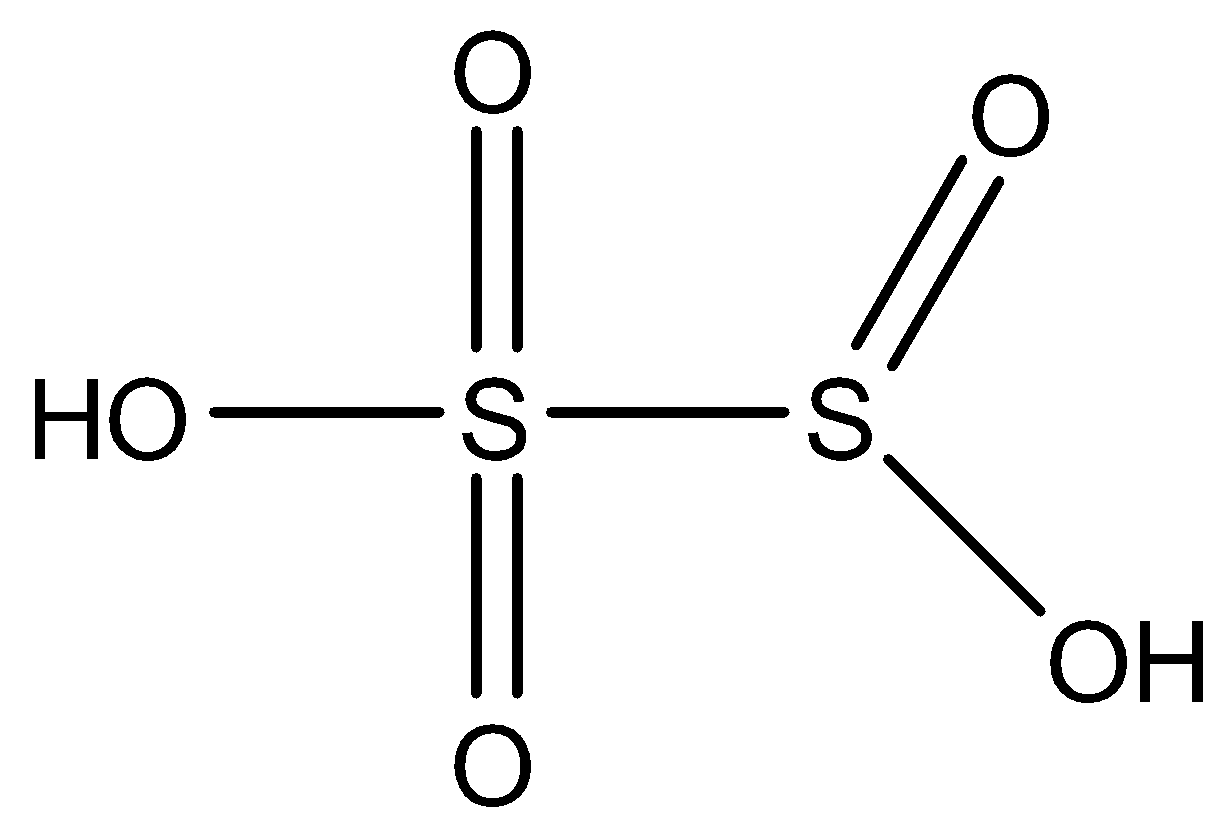
${{H}_{2}}{{S}_{2}}{{O}_{5}}$: This compound is known as Disulfurous acid. In this compound, two sulfur atoms are joined together with a single bond. There are three double bond oxygen and two $OH$ bonds. The structure is:
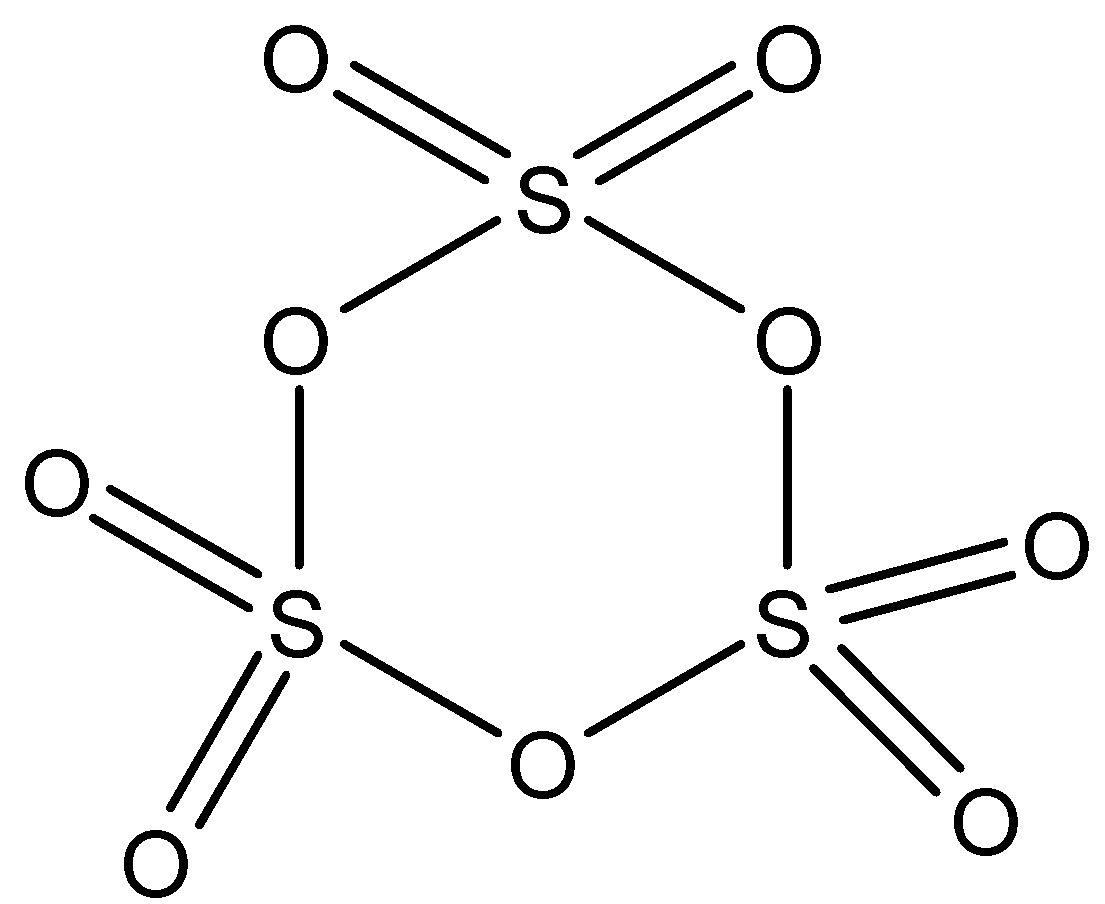
${{S}_{3}}{{O}_{9}}$: This compound is known as sulfur trioxide (orth). This compound has three $S-O-S$ bonds and each sulfur atom is further attached to two oxygen atoms by a double bond. The structure is:
${{S}_{2}}O_{6}^{2-}$: This compound is known as the conjugate acid of Dithionic acid. In this compound, two sulfur atoms are joined together with a single bond. Both the sulfur is attached to two oxygen atoms with a double bond and one oxygen atom with a single bond has a negative charge. The structure is:

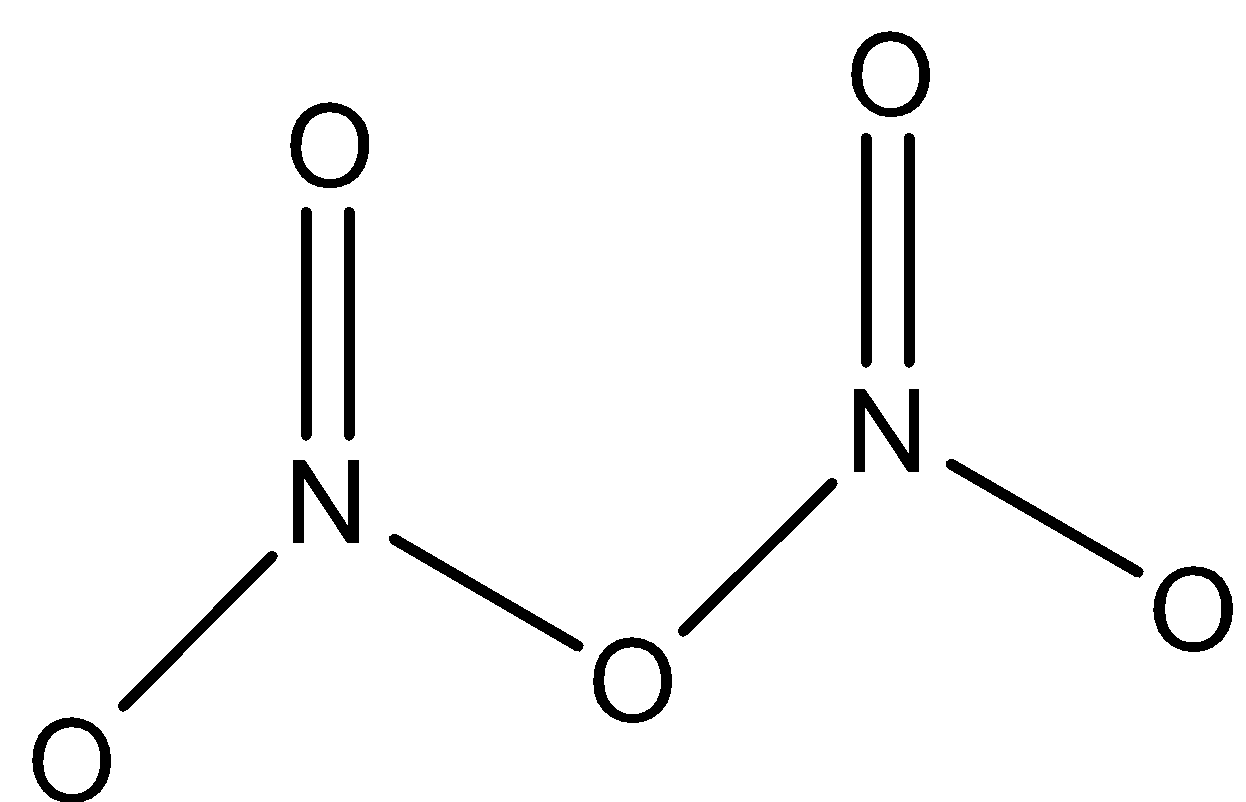
${{N}_{2}}{{O}_{5}}$: This compound is known as dinitrogen pentoxide. This compound has one $N-O-N$ bond. And both nitrogen atoms are joined to two oxygen atoms. The structure is:
${{N}_{2}}O$: This compound is known as nitrous oxide. The structure is given below:

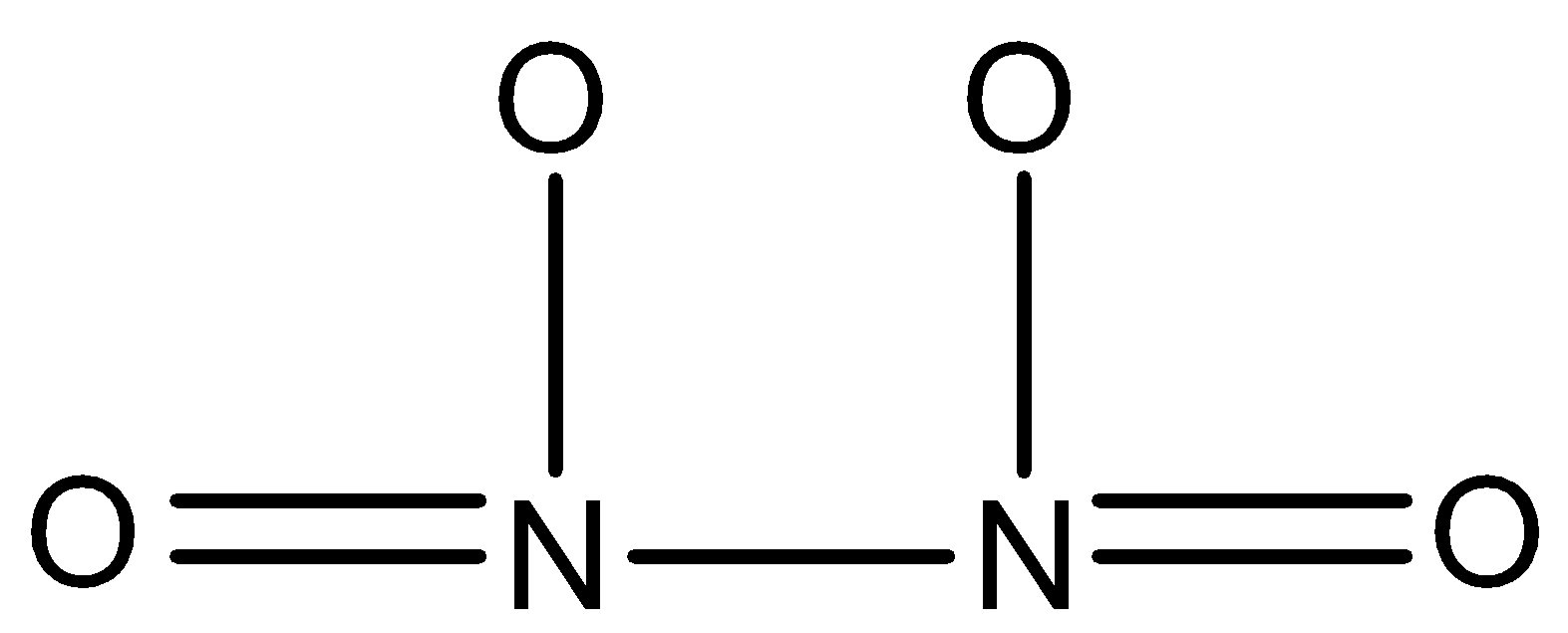
${{N}_{2}}{{O}_{4}}$: This compound is known as dinitrogen tetroxide. In this, two nitrogen atoms are joined together by a single bond and both nitrogen atoms are joined to two oxygen atoms. The structure is:
${{P}_{4}}{{O}_{10}}$: This compound is known as phosphorus pentoxide. There are six $P-O-P$ bonds in this structure and phosphorus is further bonded to one oxygen atom. The structure is:
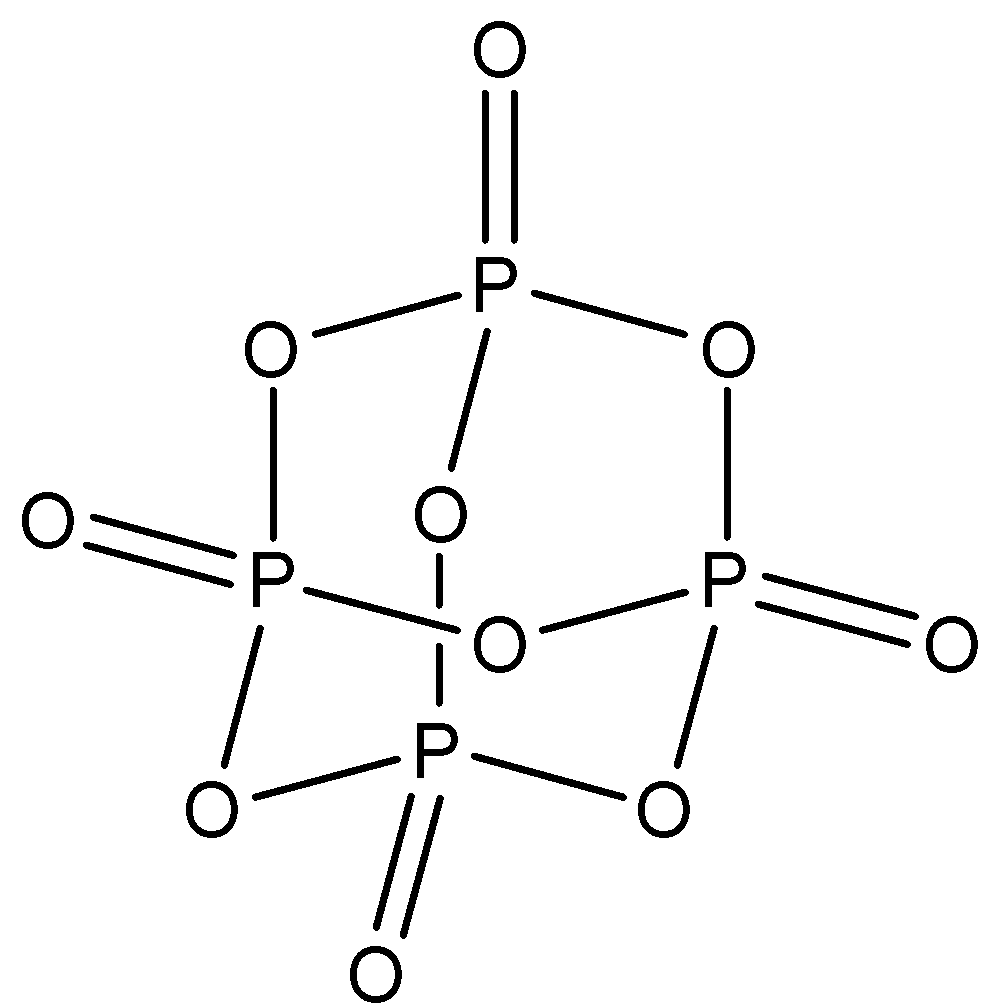
${{P}_{4}}{{O}_{6}}$: This compound is known as phosphorus trioxide. There are six $P-O-P$ bonds in this structure. The structure is:
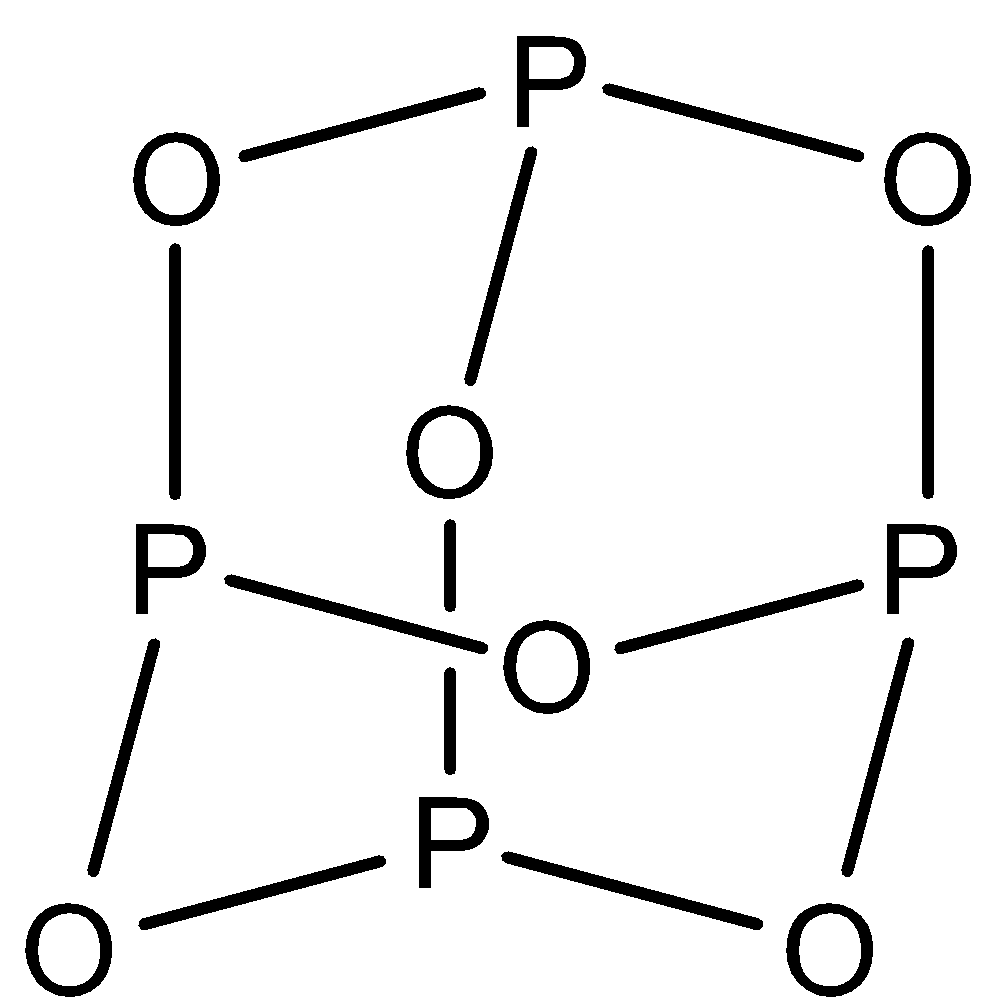
${{H}_{5}}{{P}_{3}}{{O}_{10}}$: This compound is known as triphosphoric acid. This compound has three $P-O-P$ bonds. The structure is given below:
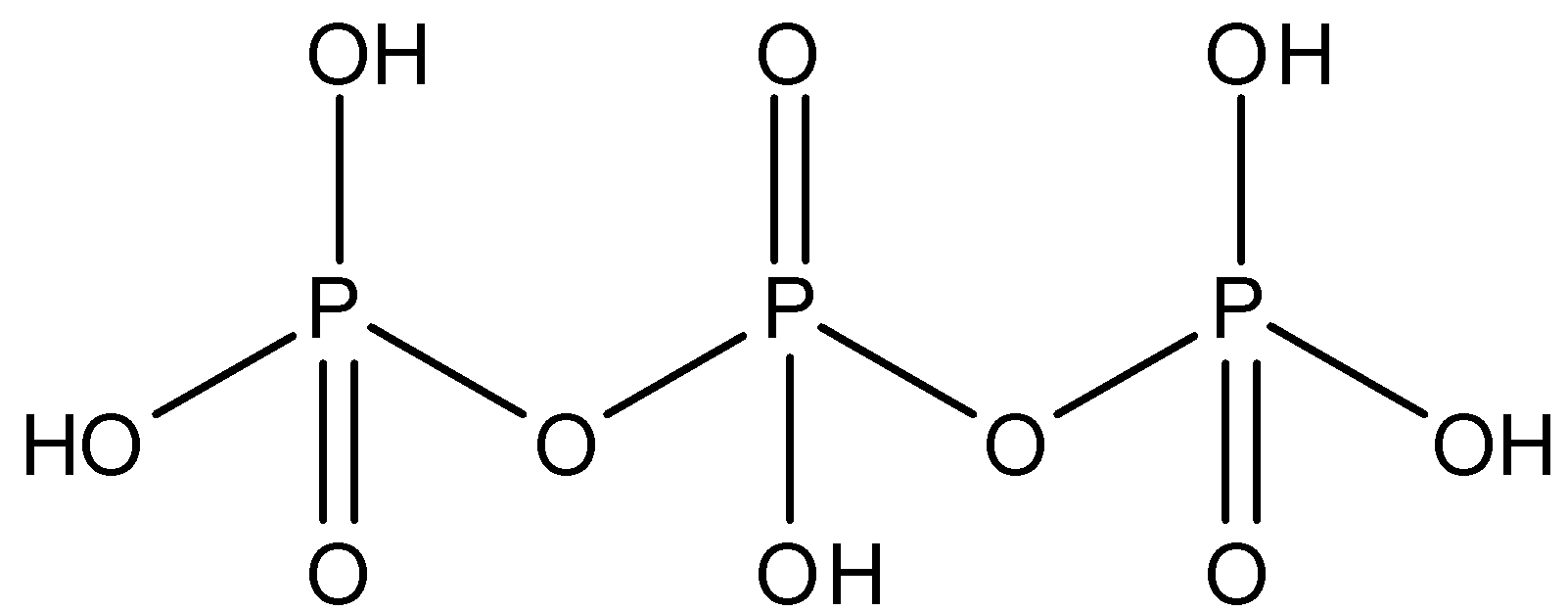
$S{{i}_{2}}O_{7}^{6-}$: This compound is known as disilicate. This compound has one $Si-O-Si$ bond. The structure is given below:
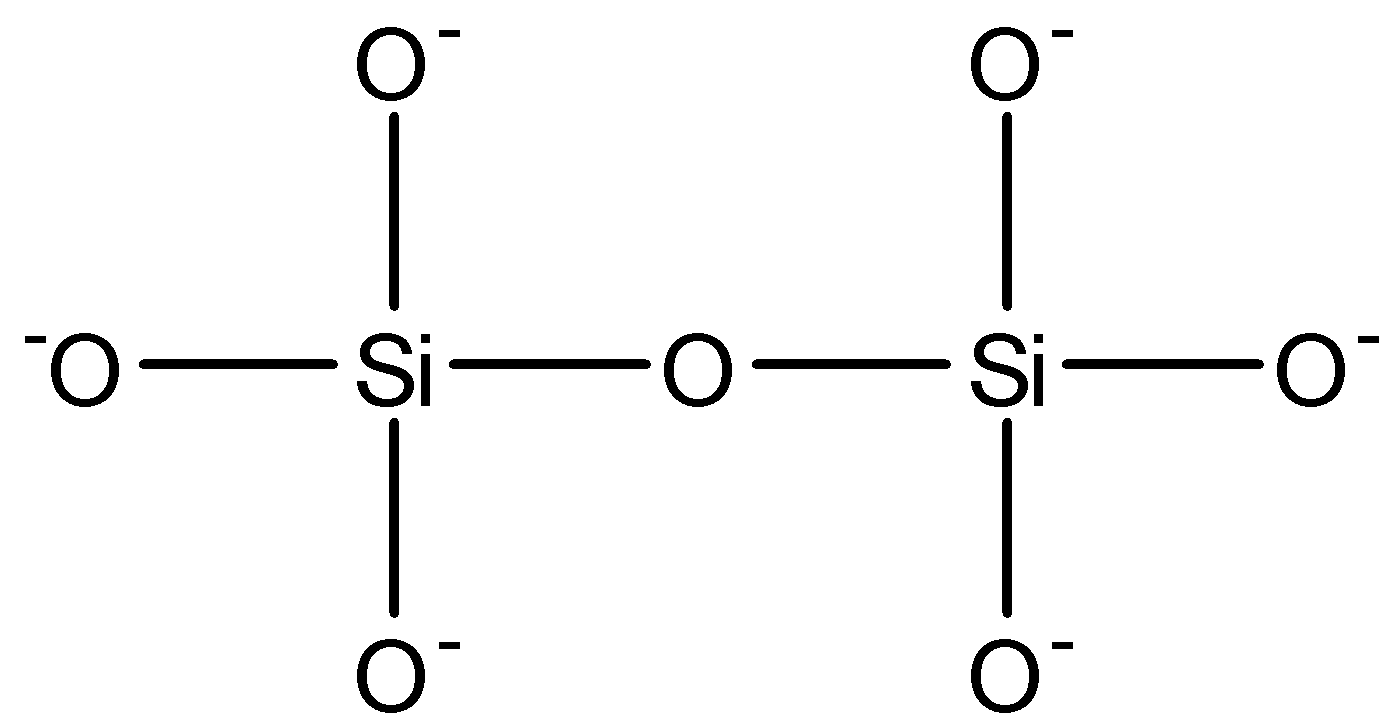
From the above discussion option (b) and option (d) are correct.
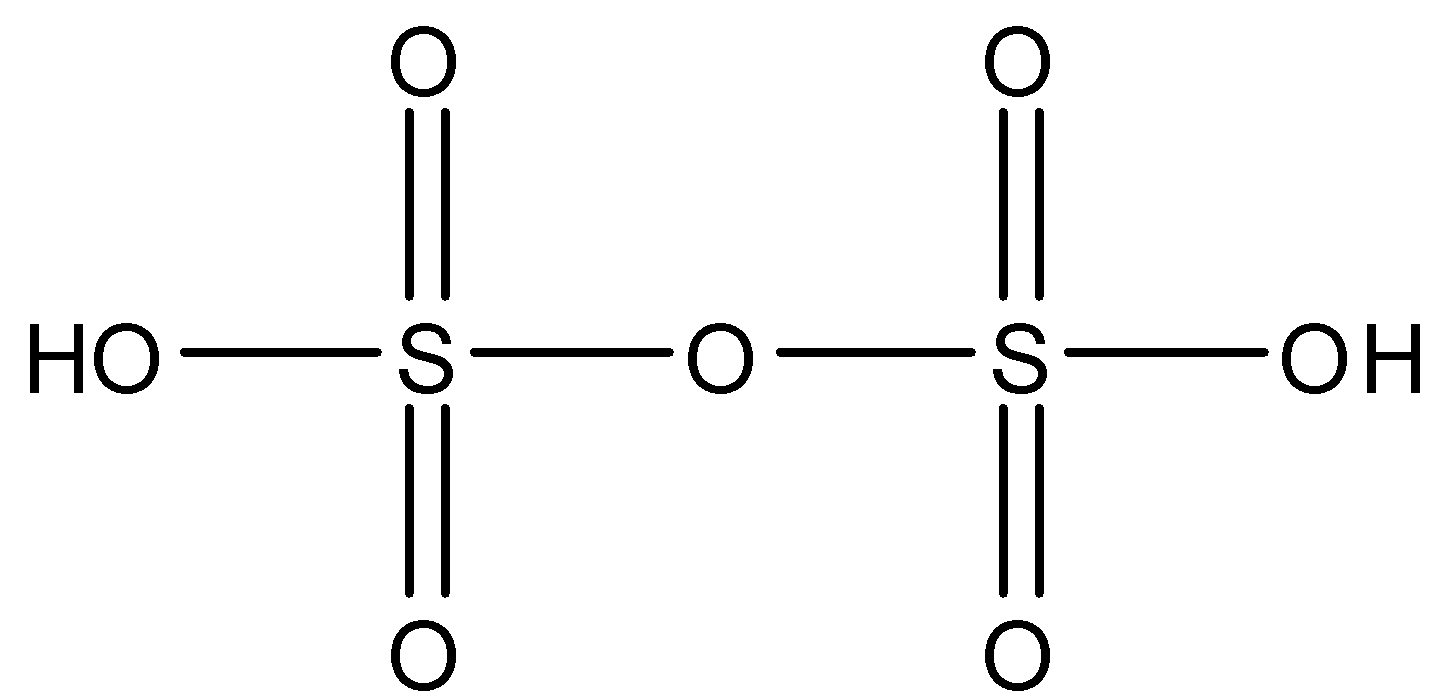
Note: All the oxoacids are mostly acidic. Other compounds that have a $X-O-X$ bond is ${{H}_{2}}{{S}_{2}}{{O}_{7}}$. This is known as Oleum and its structure is given below:
Complete step by step answer:
All the compounds given in the above options are oxoacids of sulfur, phosphorus, nitrogen, and silicon. Let us see the structure of all the compounds in the given options.
${{H}_{2}}{{S}_{2}}{{O}_{5}}$: This compound is known as Disulfurous acid. In this compound, two sulfur atoms are joined together with a single bond. There are three double bond oxygen and two $OH$ bonds. The structure is:

${{S}_{3}}{{O}_{9}}$: This compound is known as sulfur trioxide (orth). This compound has three $S-O-S$ bonds and each sulfur atom is further attached to two oxygen atoms by a double bond. The structure is:

${{S}_{2}}O_{6}^{2-}$: This compound is known as the conjugate acid of Dithionic acid. In this compound, two sulfur atoms are joined together with a single bond. Both the sulfur is attached to two oxygen atoms with a double bond and one oxygen atom with a single bond has a negative charge. The structure is:

${{N}_{2}}{{O}_{5}}$: This compound is known as dinitrogen pentoxide. This compound has one $N-O-N$ bond. And both nitrogen atoms are joined to two oxygen atoms. The structure is:

${{N}_{2}}O$: This compound is known as nitrous oxide. The structure is given below:

${{N}_{2}}{{O}_{4}}$: This compound is known as dinitrogen tetroxide. In this, two nitrogen atoms are joined together by a single bond and both nitrogen atoms are joined to two oxygen atoms. The structure is:

${{P}_{4}}{{O}_{10}}$: This compound is known as phosphorus pentoxide. There are six $P-O-P$ bonds in this structure and phosphorus is further bonded to one oxygen atom. The structure is:

${{P}_{4}}{{O}_{6}}$: This compound is known as phosphorus trioxide. There are six $P-O-P$ bonds in this structure. The structure is:

${{H}_{5}}{{P}_{3}}{{O}_{10}}$: This compound is known as triphosphoric acid. This compound has three $P-O-P$ bonds. The structure is given below:

$S{{i}_{2}}O_{7}^{6-}$: This compound is known as disilicate. This compound has one $Si-O-Si$ bond. The structure is given below:

From the above discussion option (b) and option (d) are correct.
Note: All the oxoacids are mostly acidic. Other compounds that have a $X-O-X$ bond is ${{H}_{2}}{{S}_{2}}{{O}_{7}}$. This is known as Oleum and its structure is given below:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




