
In what respects does lithium hydrogen carbonate differ from other alkali metal hydrogen carbonates?
Answer
504.3k+ views
Hint: Lithium carbonate decomposes on heating to the metal oxide and carbon dioxide, but the rest of the group one carbonates do not decompose at laboratory temperatures, although at higher temperature this becomes possible. The decomposition temperature again increases down the group.
Complete answer:
Lithium hydrogen carbonate $(LiHC{O_3})$ is found in solution while the rest of alkali hydrogen carbonates are solid as they are so unstable to heat that they only exist in solution. Lithium Is small in size and has high polarizing power.
The molecular structure of carbonate is given below:
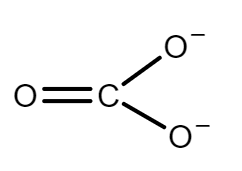
This figure shows two carbon-oxygen single bonds and one double bond, with two oxygen atoms each carrying a negative charge. The charges are delocalized. Imagine that a carbonate ion is placed next to a positive ion. The positive ion attracts the delocalized electrons in the carbonate ion towards itself. The carbonate ion becomes polarized. If this system is heated, the carbon dioxide breaks free, leaving a metal oxide.
The amount of heat required depends on how polarized the ion is, if it is highly polarized, less heat is required than if it is only slightly polarized. If the positive ion only has one positive charge, the polarizing effect is lessened. The smaller the positive ion, the higher the charge density, and the greater the effect on the carbonate ion. As the positive ions get bigger down the group, they have less effect on the carbonate ions near them, the compound must be heated more in order to force the carbon dioxide to break off and leave the metal oxide.
Note:
The group $1$ is more thermally stable than those in group $2$ because, group $1$ compounds must be heated more because the carbonate ion is less polarized by a singly charged positive ion. In other words, carbonate becomes more thermally stable down the group. The group one hydrogen carbonate is stable enough to exist as solid, although it decomposes easily on heating.
Complete answer:
Lithium hydrogen carbonate $(LiHC{O_3})$ is found in solution while the rest of alkali hydrogen carbonates are solid as they are so unstable to heat that they only exist in solution. Lithium Is small in size and has high polarizing power.
The molecular structure of carbonate is given below:

This figure shows two carbon-oxygen single bonds and one double bond, with two oxygen atoms each carrying a negative charge. The charges are delocalized. Imagine that a carbonate ion is placed next to a positive ion. The positive ion attracts the delocalized electrons in the carbonate ion towards itself. The carbonate ion becomes polarized. If this system is heated, the carbon dioxide breaks free, leaving a metal oxide.
The amount of heat required depends on how polarized the ion is, if it is highly polarized, less heat is required than if it is only slightly polarized. If the positive ion only has one positive charge, the polarizing effect is lessened. The smaller the positive ion, the higher the charge density, and the greater the effect on the carbonate ion. As the positive ions get bigger down the group, they have less effect on the carbonate ions near them, the compound must be heated more in order to force the carbon dioxide to break off and leave the metal oxide.
Note:
The group $1$ is more thermally stable than those in group $2$ because, group $1$ compounds must be heated more because the carbonate ion is less polarized by a singly charged positive ion. In other words, carbonate becomes more thermally stable down the group. The group one hydrogen carbonate is stable enough to exist as solid, although it decomposes easily on heating.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




