
In the given reaction,
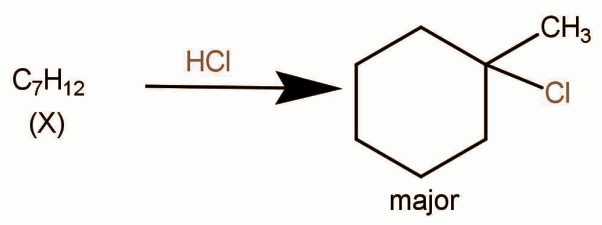
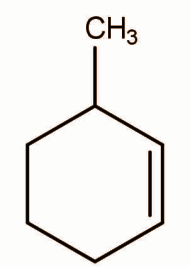
Structure of X can be:
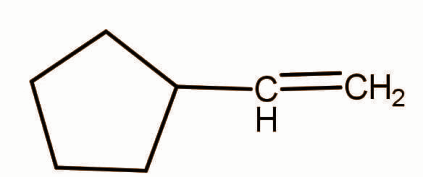
A.
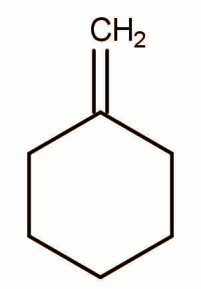
B.
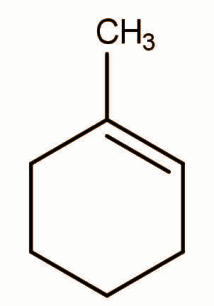
C.
D.





Answer
581.1k+ views
Hint: The above reaction is the additional reaction. The hydrogen chloride adds up to a molecule of alkene according to Markovnikov addition and carbocation rearrangement occurs if necessary to form a more stable carbocation.
Complete step by step solution:
It is clear that the reacting molecule is an alkene from the given options. The addition of \[{\text{HCl}}\] in alkene occurs from Markovnikov addition; it says that the nucleophile that is the chloride will attached to that carbon which is more highly substituted or which have lesser number of hydrogen let us write the product in each of the above reaction:
In option A a 5 member ring is given. First of all the carbocation will form at carbon next to the ring and then ring expansion occurs to form the 6 membered ring and hence chloride will react to form the given product.
In Case of option B addition to alkene will occur and the chloride will go to that carbon which is more highly substituted that is the carbon that belongs to the cyclic ring in this the product formed will be the same.
In the same way the Markovnikov addition will occur in option C and the same product will form. Chloride will go to carbon attached with the methyl group because it is highly substituted.
In the option D carbocation rearrangement will occur after addition of hydrogen because tertiary carbocation is more stable than the secondary carbocation. Hence the same product will form.
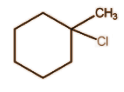
So in all of them the major product will be:
Note: First of all alkene attacks the hydrogen and hence a carbocation is formed, the carbocation is formed on the more substituted carbon and then chloride attaches with the carbocation. The electrophile which is generated, that is, the hydrogen ion attaches at the beginning, so it is known as the electrophilic addition reaction.
Complete step by step solution:
It is clear that the reacting molecule is an alkene from the given options. The addition of \[{\text{HCl}}\] in alkene occurs from Markovnikov addition; it says that the nucleophile that is the chloride will attached to that carbon which is more highly substituted or which have lesser number of hydrogen let us write the product in each of the above reaction:
In option A a 5 member ring is given. First of all the carbocation will form at carbon next to the ring and then ring expansion occurs to form the 6 membered ring and hence chloride will react to form the given product.
In Case of option B addition to alkene will occur and the chloride will go to that carbon which is more highly substituted that is the carbon that belongs to the cyclic ring in this the product formed will be the same.
In the same way the Markovnikov addition will occur in option C and the same product will form. Chloride will go to carbon attached with the methyl group because it is highly substituted.
In the option D carbocation rearrangement will occur after addition of hydrogen because tertiary carbocation is more stable than the secondary carbocation. Hence the same product will form.
So in all of them the major product will be:

Note: First of all alkene attacks the hydrogen and hence a carbocation is formed, the carbocation is formed on the more substituted carbon and then chloride attaches with the carbocation. The electrophile which is generated, that is, the hydrogen ion attaches at the beginning, so it is known as the electrophilic addition reaction.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




