
In the given below reaction,find the product?
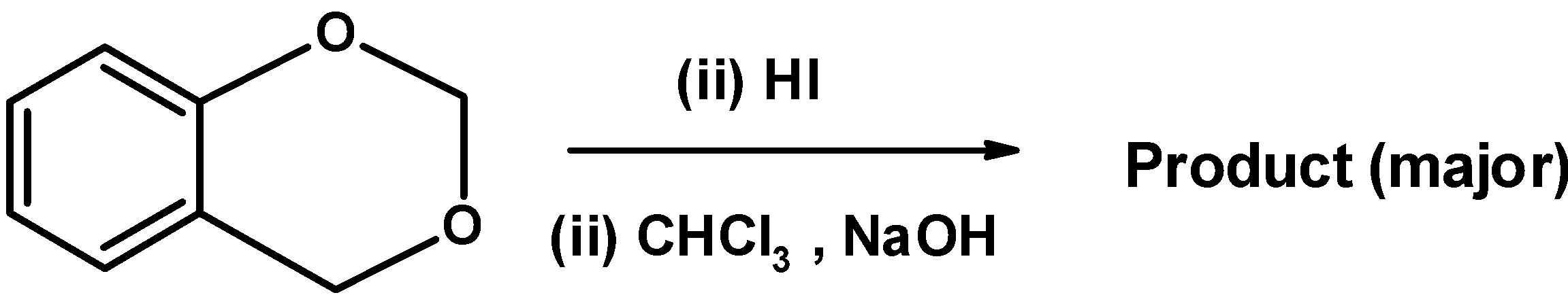
A)
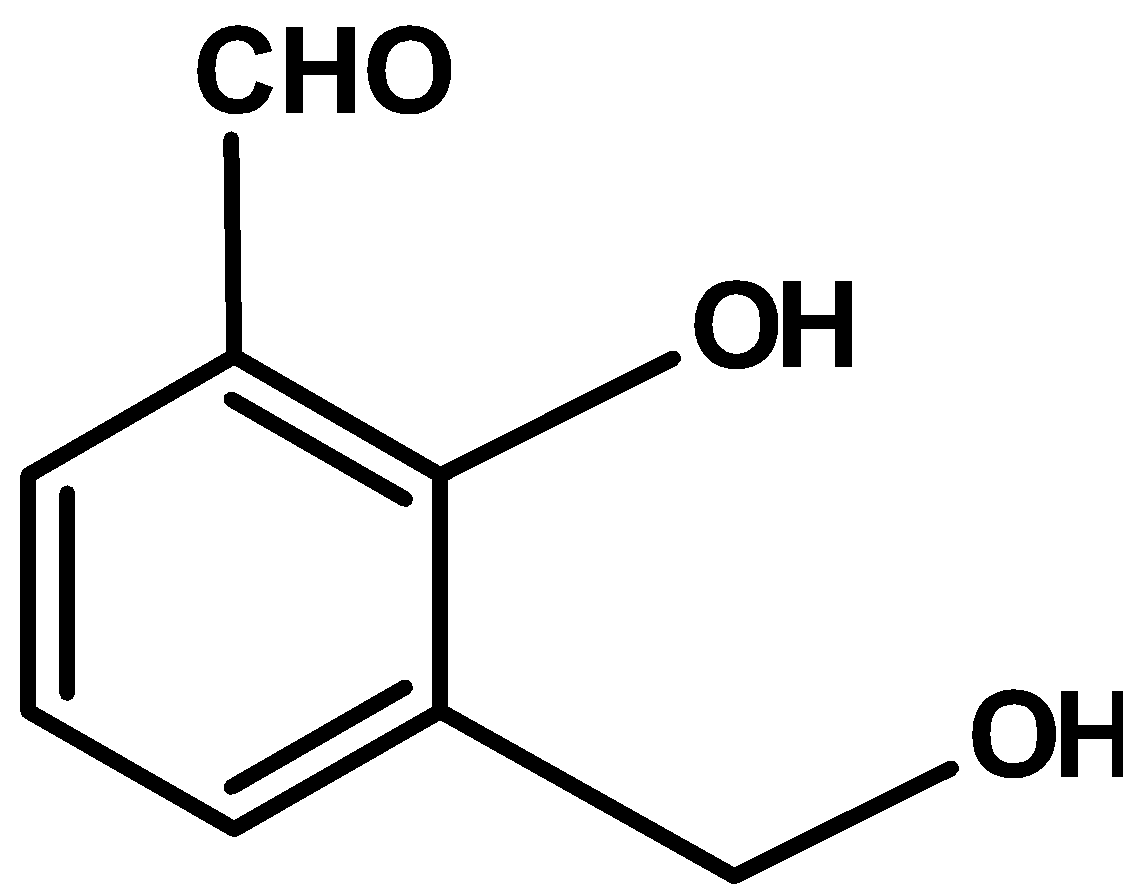
B)
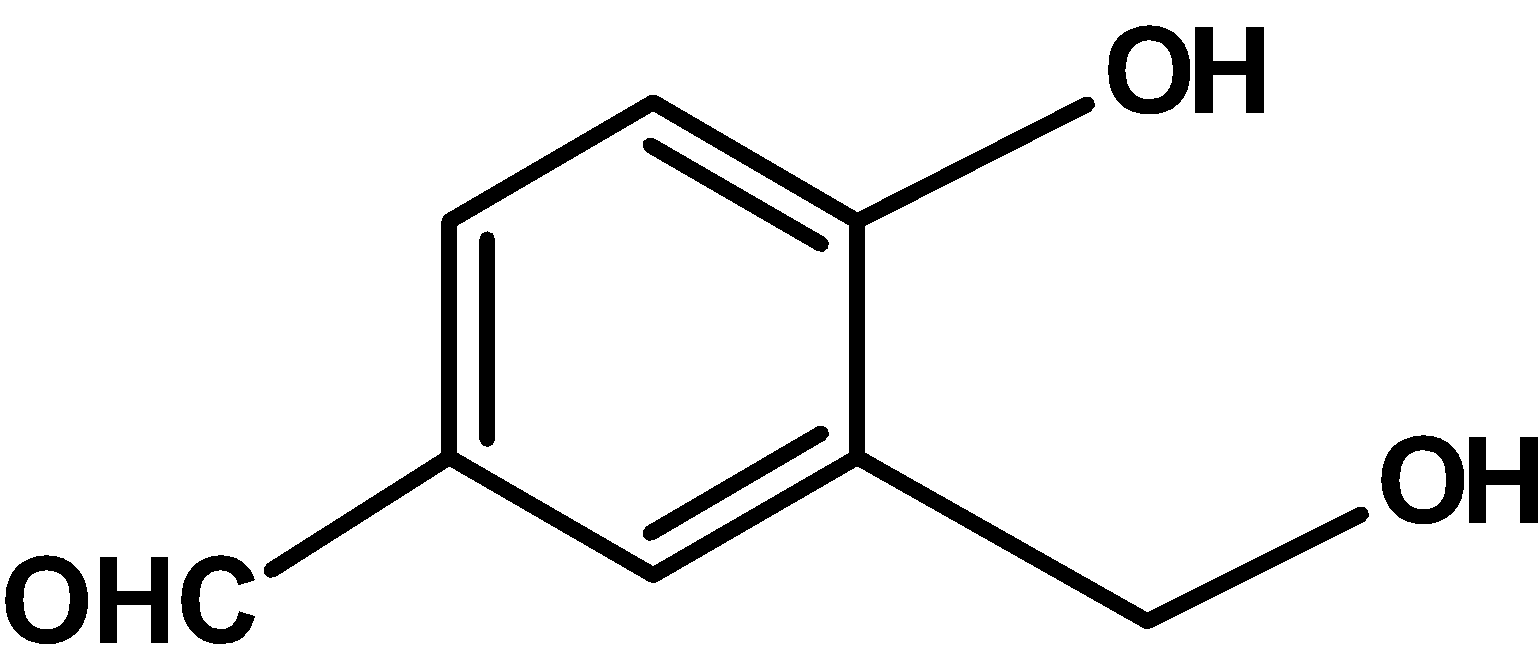
C)
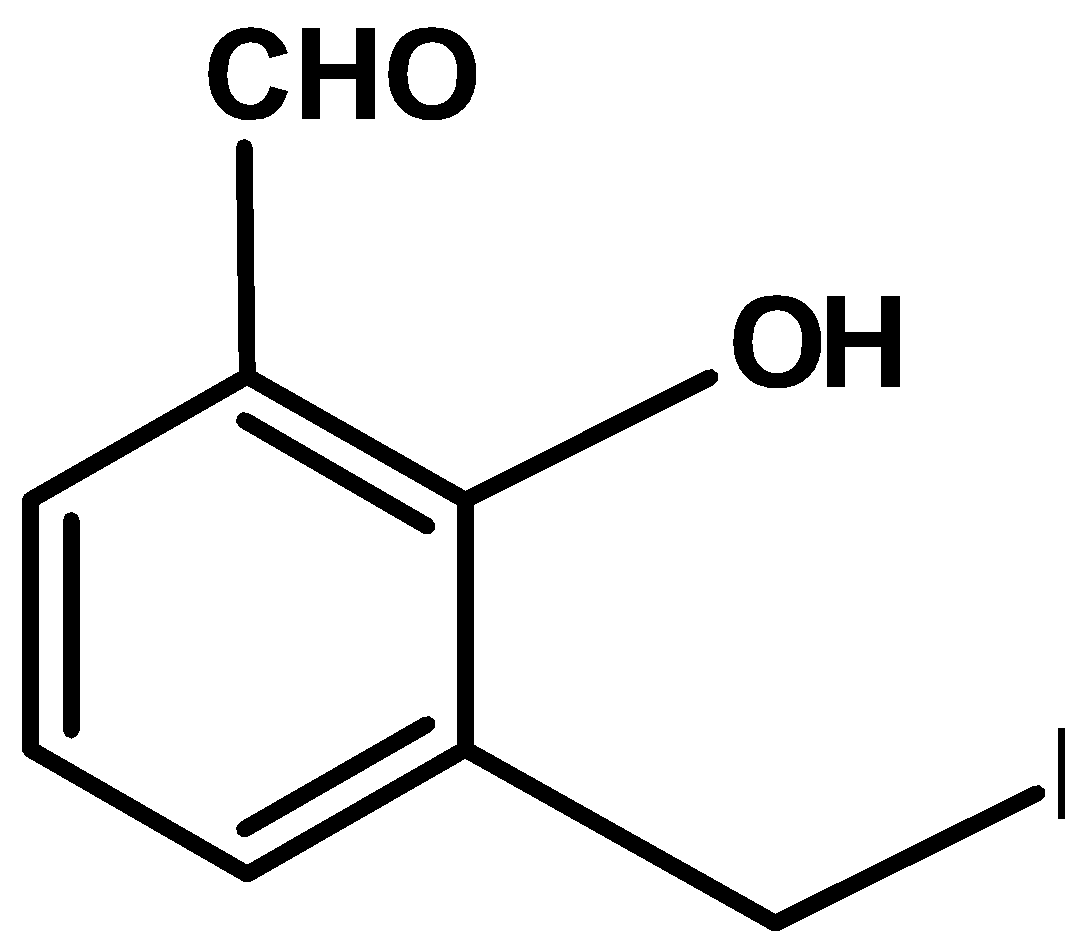
D)
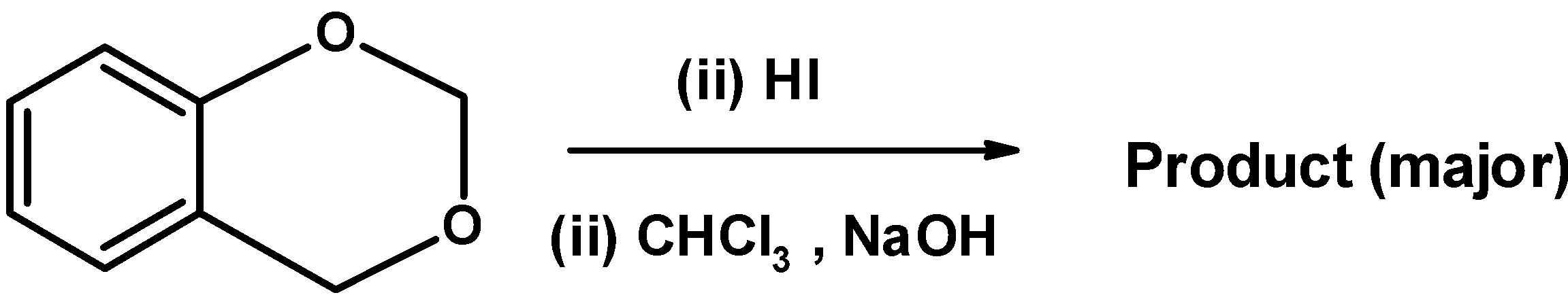
| A) | 
|
| B) | 
|
| C) | 
|
| D) | 
|
Answer
577.5k+ views
Hint: When phenol reacts is refluxed with chloroform in the presence of an aqueous solution of sodium hydroxide at $\text{ 340 K }$ followed by the hydrolysis, an aldehydic group gets introduced in the ring at a position ortho to the phenolic group. This reaction is a Reimer –Tiemann reaction.
Complete answer:
Here, we have been interested to find out the major product of the reaction.
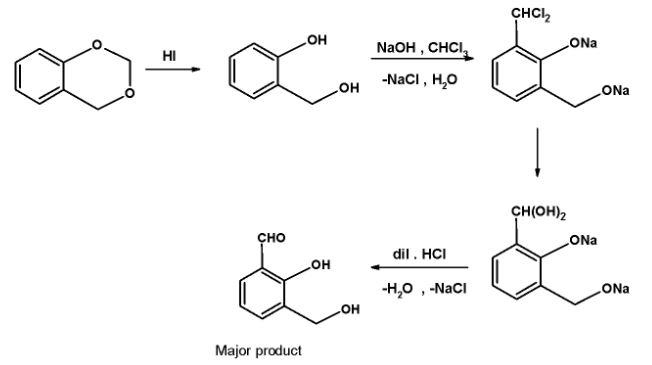
In the first step the hydrogen iodide $\text{ HI }$ reagent act on the compound. The halide (iodide ) attacks on one of the oxygen atoms in such a manner that the bond holding two oxygen atoms breaks leaving behind the formation of hydroxyl on either end.
The action of hydrogen iodide from the two hydroxyls.
The next step involves the action of chloroform in aqueous sodium hydroxide on the phenol. When phenol reacts is refluxed with chloroform in the presence of an aqueous solution of sodium hydroxide at $\text{ 340 K }$ followed by the hydrolysis, an aldehydic group gets introduced in the ring at a position ortho to the phenolic group. This reaction is a Reimer –Tiemann reaction.
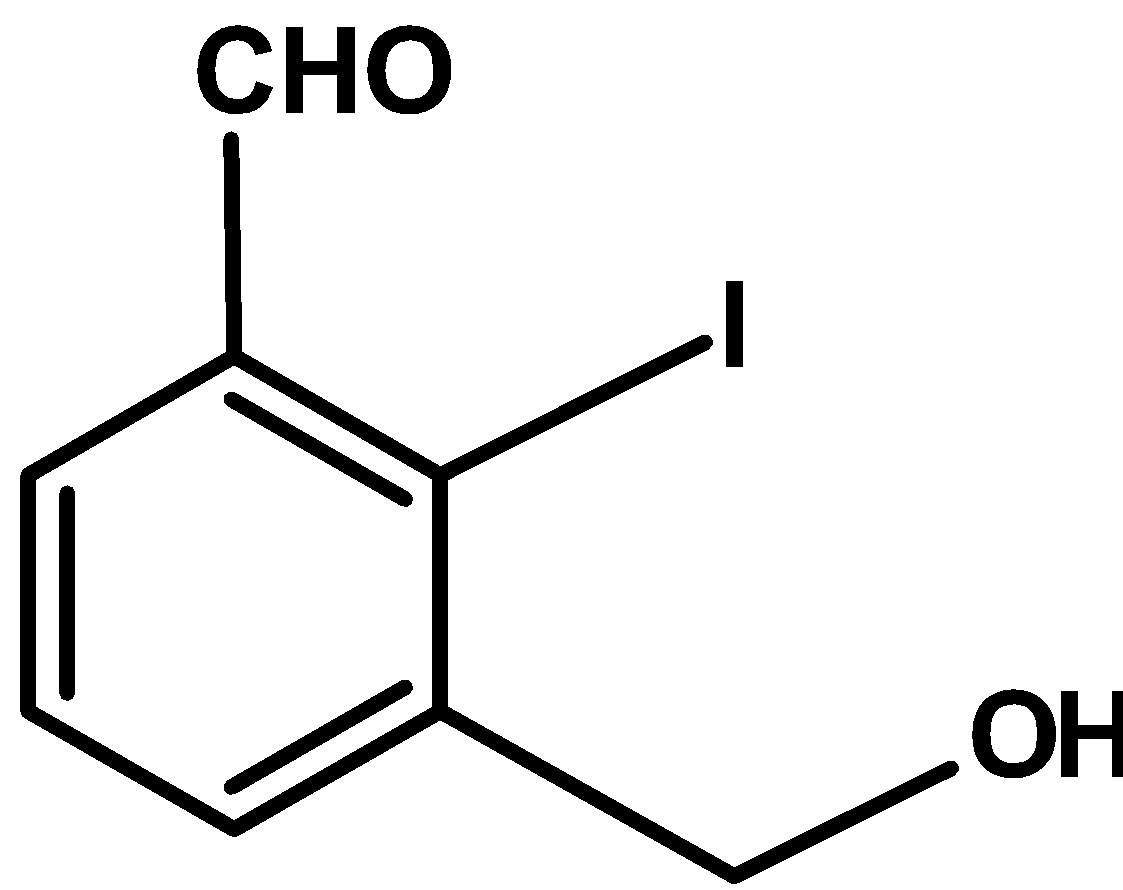
This is a Reimer Tiemann reaction. Here, the ortho position next to the hydroxyl group (directly bonded to the ring) is substituted with the aldehydic group. Only one position is available for the addition of aldehydic groups. Thus obtained product is as shown below,
Hence, (A) is the correct option.
Note:
Note that, in reimer Tiemann reaction in addition to ortho product, a small amount of para product is also formed, but is it in very small amount thus ortho product is considered as the major product. Thus here, in spite of having a para product the major product would be the ortho.
Complete answer:
Here, we have been interested to find out the major product of the reaction.
In the first step the hydrogen iodide $\text{ HI }$ reagent act on the compound. The halide (iodide ) attacks on one of the oxygen atoms in such a manner that the bond holding two oxygen atoms breaks leaving behind the formation of hydroxyl on either end.
The action of hydrogen iodide from the two hydroxyls.
The next step involves the action of chloroform in aqueous sodium hydroxide on the phenol. When phenol reacts is refluxed with chloroform in the presence of an aqueous solution of sodium hydroxide at $\text{ 340 K }$ followed by the hydrolysis, an aldehydic group gets introduced in the ring at a position ortho to the phenolic group. This reaction is a Reimer –Tiemann reaction.
This is a Reimer Tiemann reaction. Here, the ortho position next to the hydroxyl group (directly bonded to the ring) is substituted with the aldehydic group. Only one position is available for the addition of aldehydic groups. Thus obtained product is as shown below,

Hence, (A) is the correct option.
Note:
Note that, in reimer Tiemann reaction in addition to ortho product, a small amount of para product is also formed, but is it in very small amount thus ortho product is considered as the major product. Thus here, in spite of having a para product the major product would be the ortho.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




