
In the formation of compound (E) from (A), the name of the reaction is:
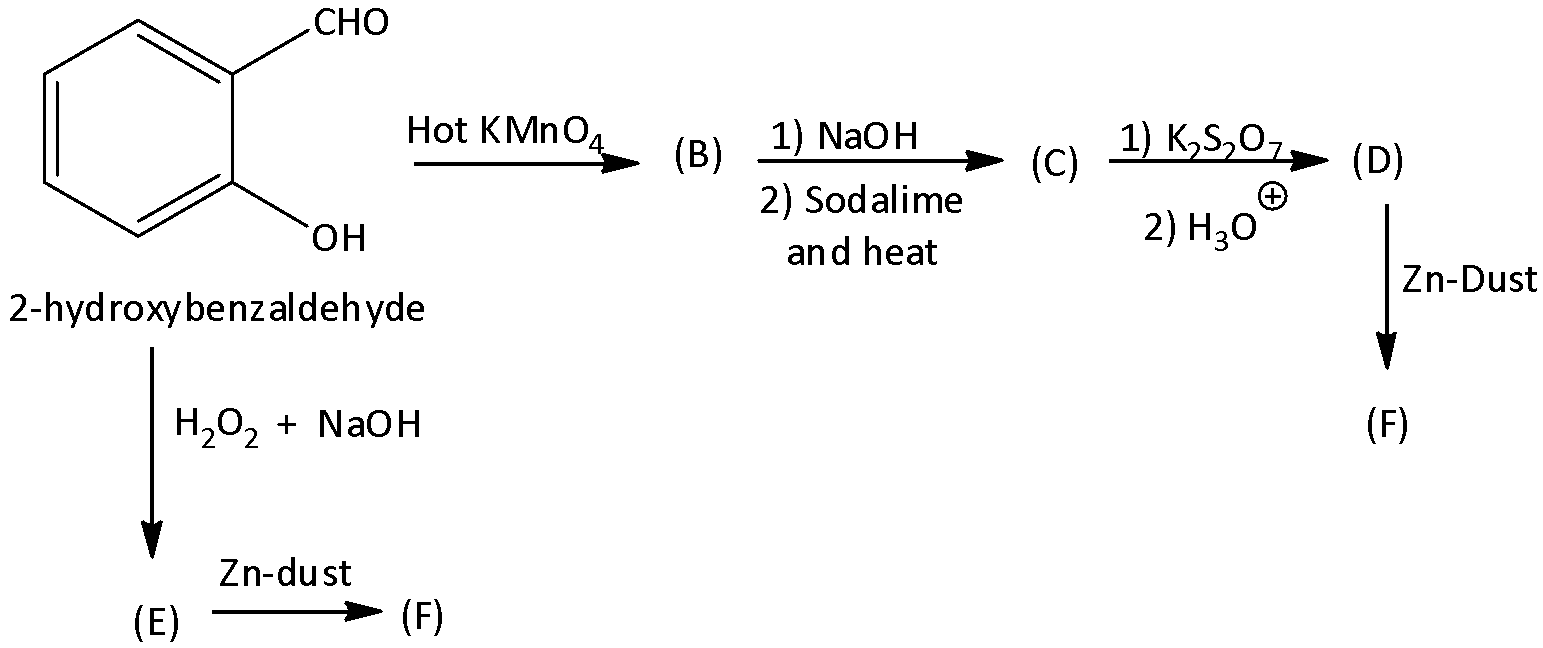
A. Elbs persulfate oxidation
B. Mannich reaction
C. Dakin’s reaction
D. Oppenauer oxidation

Answer
582.9k+ views
Hint: When we oxidize aryl aldehydes (or) aryl ketones by oxidation in the presence of base with hydrogen peroxide, we get phenols as the product.
Complete step by step answer: Let us now complete the reaction,
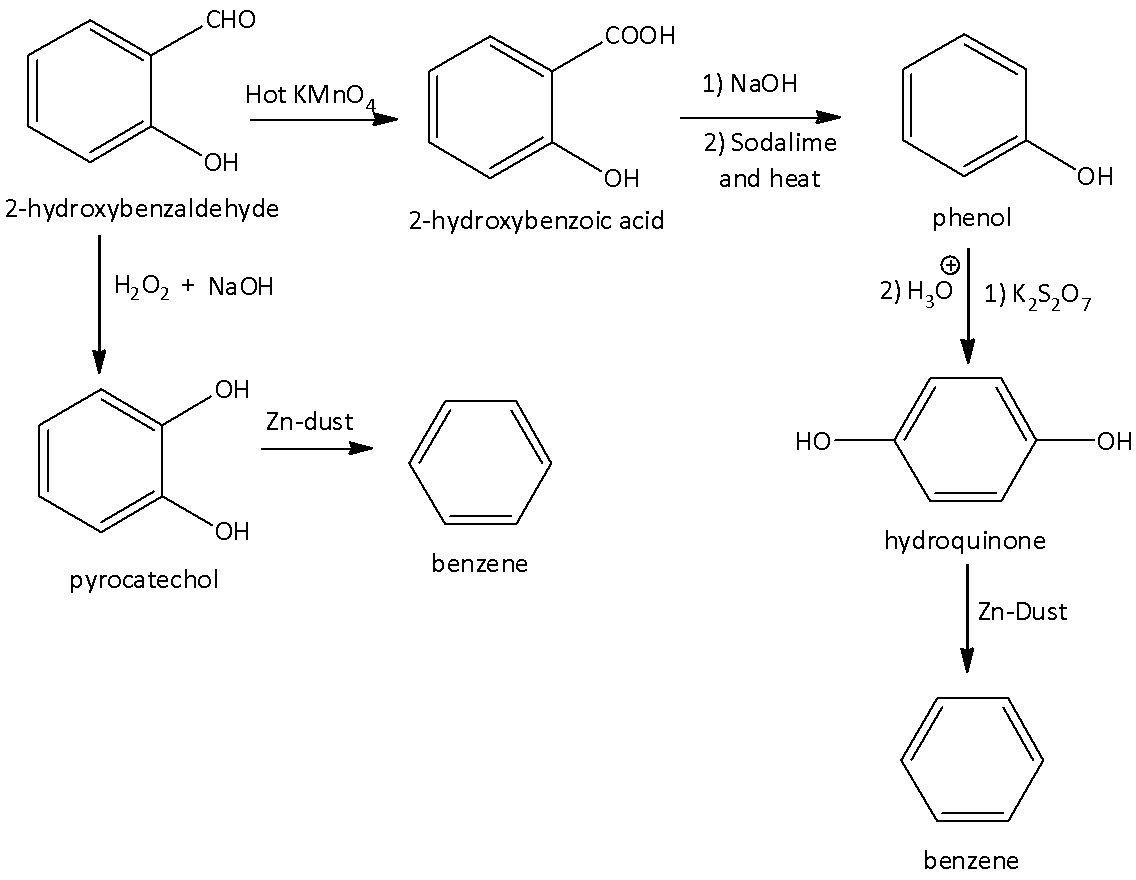
We can say the Elbs ' persulfate reaction is a reaction which converts phenols to para-diphenols in the presence of potassium persulfate. The reaction starts with nucleophilic displacement on the peroxide oxygen of the peroxydisulfate ion to form an intermediate sulfate group that is hydrolyzed to the hydroxyl group. The conversion of phenol to hydroquinone (C to D) is Elbs persulfate reaction.
Therefore, Option (A) is incorrect.
A reaction that has amino alkylation of an acidic proton placed next to carbonyl group by formaldehyde and ammonia (or) a primary (or) secondary amine to form beta-amino carbonyl compound is known as Mannich reaction, and the final product obtained is known as Mannich base.
Therefore, Option (B) is incorrect.
Phenols can be obtained from aryl aldehydes or aryl ketones through oxidizing of hydrogen peroxide using a base. Such an organic reaction is known as Dakin reaction. The conversion of 2-hydroxybenzaldehyde to pyrocatechol (A to E) is Dakin reaction.
Therefore, Option (C) is correct.
A method for selectively oxidizing secondary alcohols to ketones is done by Oppenauer oxidation. Even though we can oxidize primary alcohols under Oppenauer conditions, primary alcohols are rarely oxidized by Oppenauer oxidation due to the competing aldol condensation of aldehyde products.
Therefore, Option (D) is incorrect.
Therefore, Option (C) is correct.
Note: We can use Dakin oxidation to prepare benzenediols and alkoxyphenols. Catechol is synthesized from o-hydroxy and o-alkoxy phenyl aldehydes and ketones. Dakin oxidation is used in the preparation of indole quinones.
Complete step by step answer: Let us now complete the reaction,

We can say the Elbs ' persulfate reaction is a reaction which converts phenols to para-diphenols in the presence of potassium persulfate. The reaction starts with nucleophilic displacement on the peroxide oxygen of the peroxydisulfate ion to form an intermediate sulfate group that is hydrolyzed to the hydroxyl group. The conversion of phenol to hydroquinone (C to D) is Elbs persulfate reaction.
Therefore, Option (A) is incorrect.
A reaction that has amino alkylation of an acidic proton placed next to carbonyl group by formaldehyde and ammonia (or) a primary (or) secondary amine to form beta-amino carbonyl compound is known as Mannich reaction, and the final product obtained is known as Mannich base.
Therefore, Option (B) is incorrect.
Phenols can be obtained from aryl aldehydes or aryl ketones through oxidizing of hydrogen peroxide using a base. Such an organic reaction is known as Dakin reaction. The conversion of 2-hydroxybenzaldehyde to pyrocatechol (A to E) is Dakin reaction.
Therefore, Option (C) is correct.
A method for selectively oxidizing secondary alcohols to ketones is done by Oppenauer oxidation. Even though we can oxidize primary alcohols under Oppenauer conditions, primary alcohols are rarely oxidized by Oppenauer oxidation due to the competing aldol condensation of aldehyde products.
Therefore, Option (D) is incorrect.
Therefore, Option (C) is correct.
Note: We can use Dakin oxidation to prepare benzenediols and alkoxyphenols. Catechol is synthesized from o-hydroxy and o-alkoxy phenyl aldehydes and ketones. Dakin oxidation is used in the preparation of indole quinones.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




