
In the following pair, which one is more basic and why?
$C{{H}_{3}}N{{H}_{2}}$ or ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$
Answer
587.7k+ views
Hint: Think about what having a basic character means with respect to electrons the electrons. Visualize the structures of both the given molecules and how the electrons act within the molecule.
Complete step by step answer:
Basicity is defined as the ability of any atom, group, or molecule to donate electrons. This is usually assumed if a molecule has atoms that has some lone pairs of electrons that can be donated. In the amino group, the lone pair that is present on the nitrogen atom is considered to contribute to the basic character of the group. Now, let us look at the structures of both the given molecules to determine which nitrogen can use the lone pair for bonding easily.
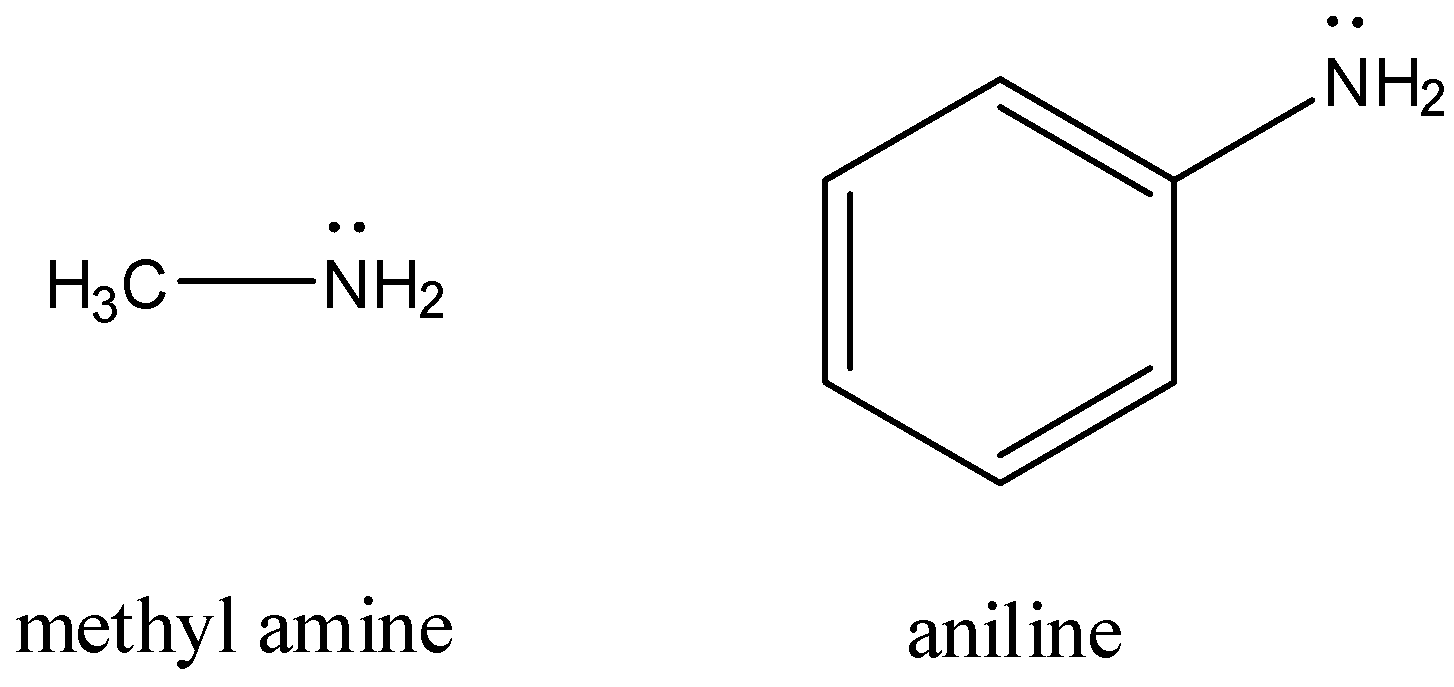
Here, methyl amine has the molecular formula $C{{H}_{3}}N{{H}_{2}}$ and aniline has the molecular formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$.
Let us consider aniline. We know that benzene has several resonating structures for stability. The lone pair that is present on the nitrogen atom in the aniline molecule delocalize and contribute to the stabilization of the aromatic ring. The nitrogen atom donates its lone pair to form a bond with the aromatic ring and gains a temporary positive charge. When this happens, it does not have any more electrons that it can donate to form any bonds which contribute to its basic character. Essentially, due to delocalization of electrons from the nitrogen atom, it loses its basic character. The resonating structures of aniline are:
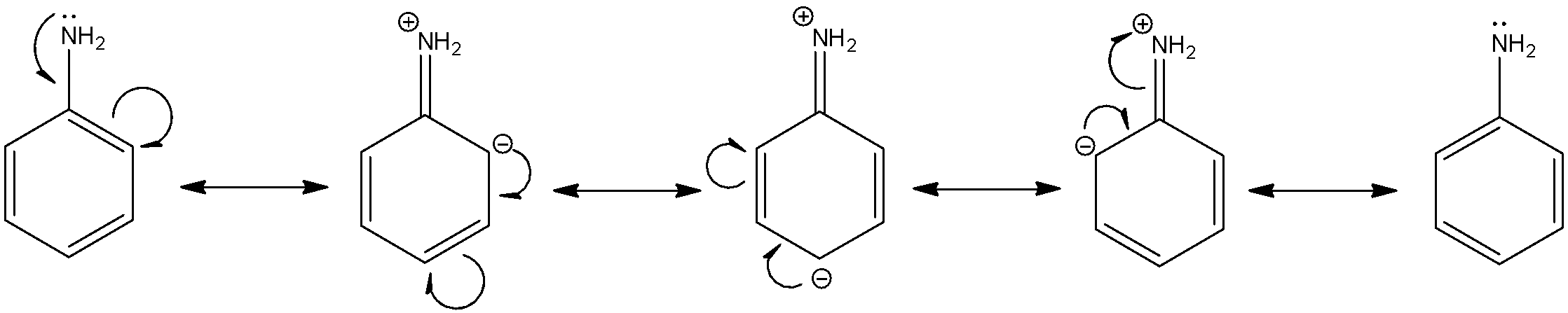
Here, we can see that the lone pair of electrons on the nitrogen atom is not available for most of the resonating structures. Thus, this reduces the basic character of aniline.
Now, let us consider the structure of methyl amine. There is no aromatic ring that requires stabilizing and thus no delocalization of the lone pair on the nitrogen atom occurs. Thus, the basic character of the amino group is retained.
Hence, methyl amine ($C{{H}_{3}}N{{H}_{2}}$) is more basic than aniline (${{C}_{6}}{{H}_{5}}N{{H}_{2}}$)
Note: Remember that the methyl group is an electron donating group. So, the electron density around the nitrogen atom increases and makes it even easier to donate the electrons.
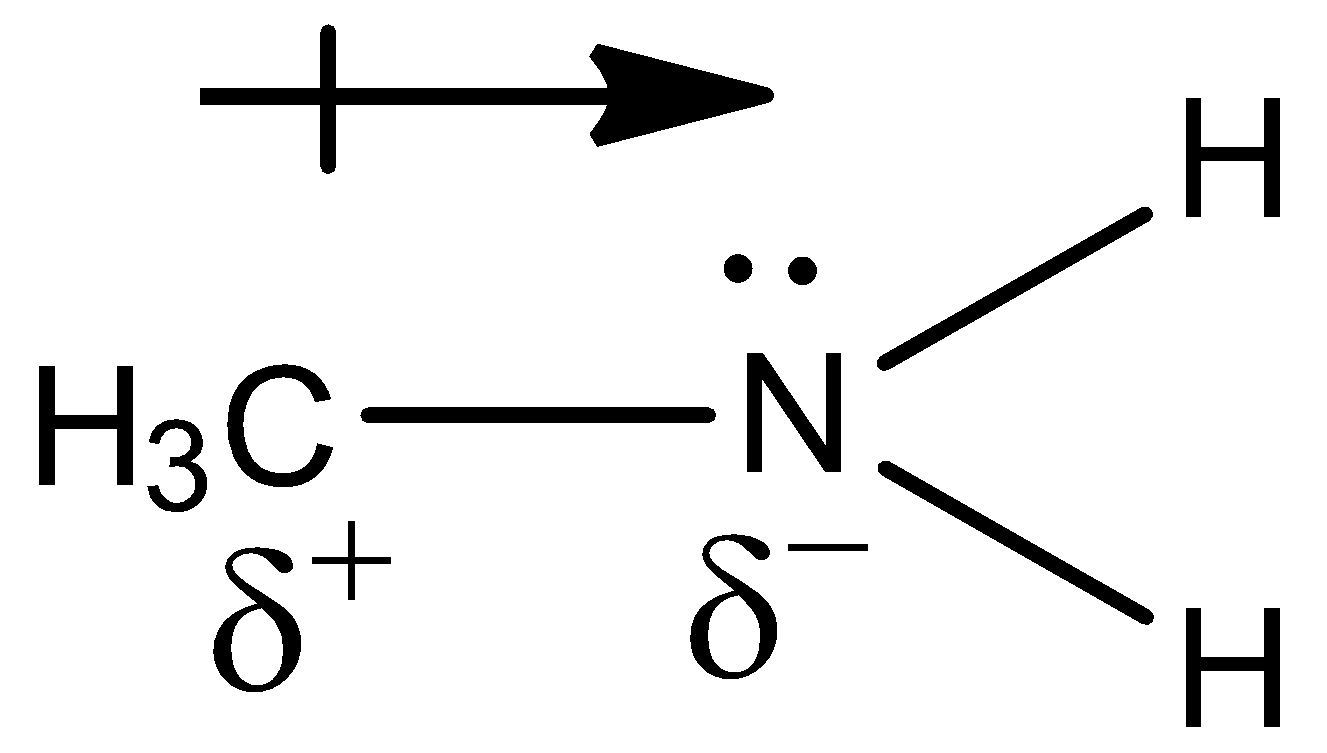
Here, we can see the partial positive and negative charges present due to the inductive effect.
Complete step by step answer:
Basicity is defined as the ability of any atom, group, or molecule to donate electrons. This is usually assumed if a molecule has atoms that has some lone pairs of electrons that can be donated. In the amino group, the lone pair that is present on the nitrogen atom is considered to contribute to the basic character of the group. Now, let us look at the structures of both the given molecules to determine which nitrogen can use the lone pair for bonding easily.

Here, methyl amine has the molecular formula $C{{H}_{3}}N{{H}_{2}}$ and aniline has the molecular formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$.
Let us consider aniline. We know that benzene has several resonating structures for stability. The lone pair that is present on the nitrogen atom in the aniline molecule delocalize and contribute to the stabilization of the aromatic ring. The nitrogen atom donates its lone pair to form a bond with the aromatic ring and gains a temporary positive charge. When this happens, it does not have any more electrons that it can donate to form any bonds which contribute to its basic character. Essentially, due to delocalization of electrons from the nitrogen atom, it loses its basic character. The resonating structures of aniline are:

Here, we can see that the lone pair of electrons on the nitrogen atom is not available for most of the resonating structures. Thus, this reduces the basic character of aniline.
Now, let us consider the structure of methyl amine. There is no aromatic ring that requires stabilizing and thus no delocalization of the lone pair on the nitrogen atom occurs. Thus, the basic character of the amino group is retained.
Hence, methyl amine ($C{{H}_{3}}N{{H}_{2}}$) is more basic than aniline (${{C}_{6}}{{H}_{5}}N{{H}_{2}}$)
Note: Remember that the methyl group is an electron donating group. So, the electron density around the nitrogen atom increases and makes it even easier to donate the electrons.

Here, we can see the partial positive and negative charges present due to the inductive effect.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




