
In the following mixed Claisen ester condensation reaction, the expected products are.
$C{H_3}COO{C_2}{H_5} + C{H_3}C{H_2}COO{C_2}{H_5}\xrightarrow[{{C_2}{H_5}OH}]{{{C_2}{H_5}ONa}}$
A) $C{H_3}COCH\left( {C{H_3}} \right)COO{C_2}{H_5}$
B)$C{H_3}C{H_2}COC{H_2}C{H_2}COO{C_2}{H_5}$
C)\[C{H_3}COC{H_2}C{H_2}COO{C_2}{H_5}\]
D) $C{H_3}C{H_2}COCH\left( {C{H_3}} \right)COO{C_2}{H_5}$
Answer
582.9k+ views
Hint:We know that the Claisen condensation is a carbon–carbon bond forming reaction between two esters or one ester and another carbonyl compound in the presence of a strong base.
Complete step by step answer:
The general reaction of Crossed Claisen reactions can be written as,
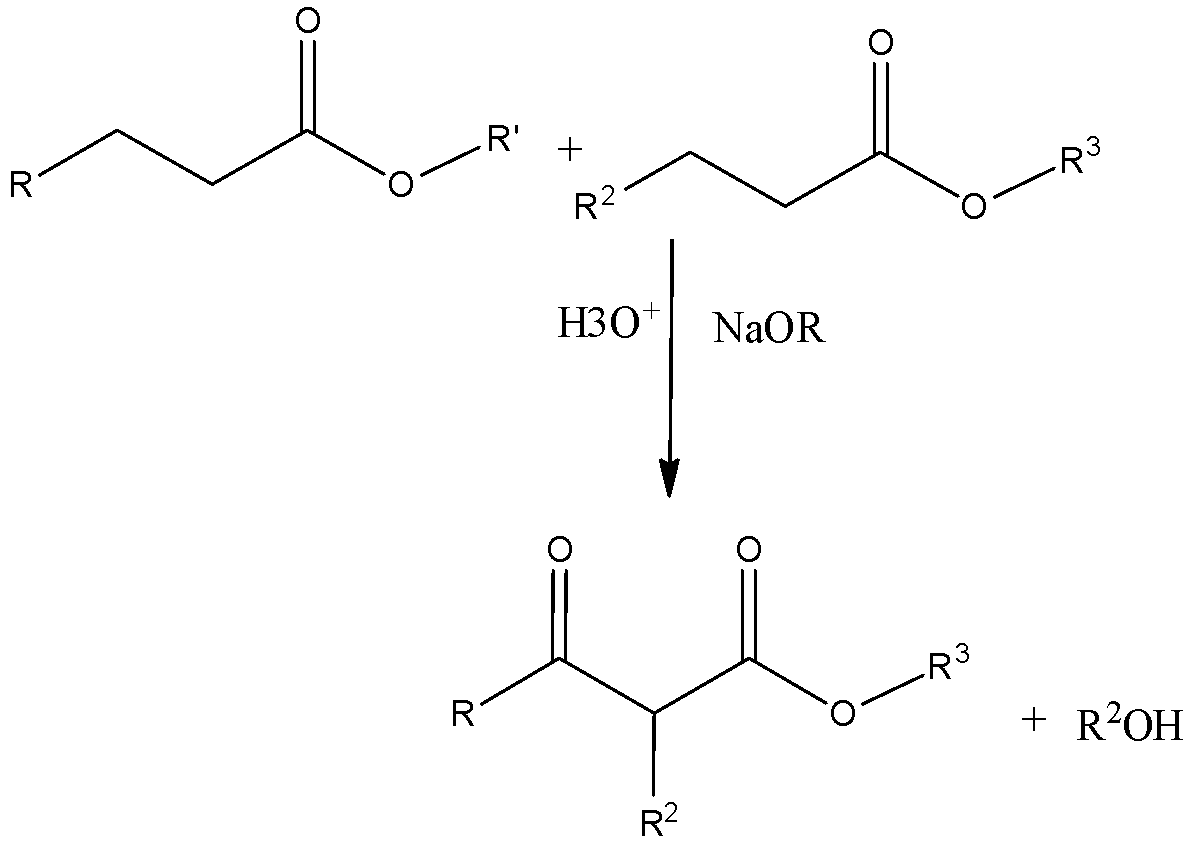
Esters having alpha hydrogen on treatment with a strong base undergo self-condensation to produce beta-keto esters. This reaction is called Claisen condensation. Mixed or crossed Claisen condensation also occurs between two different esters or between an ester and ketone.
Therefore, option B is correct.
Beta-keto ester is a reactive methylene group. When a methylene group is present between the two strongly electronegative groups such as ${\text{C = O}}$ the hydrogen atoms of the methylene group are called alpha hydrogen atoms become more acidic. The acidity of beta-keto esters depends on the stability of carbocation formed by the abstraction of alpha hydrogen. The carbanion is stabilized by resonance or by the inductive effect. The Pka value of beta keto ester is 11 and has lower pka values compared to simple ketones or esters because the additional resonance stabilizes the alternate carbonyl group. Beta-keto ester exists in keto-enol equilibrium. If the compound is symmetrical the hydrogen atom of the methylene group migrates to keto group but if the compound is unsymmetrical only one form are present and the migration of hydrogen atoms upon the inductive effect of alkyl or other groups present in either side of the methylene group. An example of beta-keto ester is acetoacetic ester.
Therefore, the option B is correct.
Note: We must remember that the alpha hydrogens of a beta keto ester exhibit acidity. In the presence of base the alpha hydrogen are abstracted and form carbanion. The acidity of the carbonyl compounds depends on the stability of carbanion formed during the reaction. More stable the carbanion more acidic the alpha hydrogen. The acidities are measured in pka vale. Lower the pka value higher is the acidity.
Complete step by step answer:
The general reaction of Crossed Claisen reactions can be written as,

Esters having alpha hydrogen on treatment with a strong base undergo self-condensation to produce beta-keto esters. This reaction is called Claisen condensation. Mixed or crossed Claisen condensation also occurs between two different esters or between an ester and ketone.
Therefore, option B is correct.
Beta-keto ester is a reactive methylene group. When a methylene group is present between the two strongly electronegative groups such as ${\text{C = O}}$ the hydrogen atoms of the methylene group are called alpha hydrogen atoms become more acidic. The acidity of beta-keto esters depends on the stability of carbocation formed by the abstraction of alpha hydrogen. The carbanion is stabilized by resonance or by the inductive effect. The Pka value of beta keto ester is 11 and has lower pka values compared to simple ketones or esters because the additional resonance stabilizes the alternate carbonyl group. Beta-keto ester exists in keto-enol equilibrium. If the compound is symmetrical the hydrogen atom of the methylene group migrates to keto group but if the compound is unsymmetrical only one form are present and the migration of hydrogen atoms upon the inductive effect of alkyl or other groups present in either side of the methylene group. An example of beta-keto ester is acetoacetic ester.
Therefore, the option B is correct.
Note: We must remember that the alpha hydrogens of a beta keto ester exhibit acidity. In the presence of base the alpha hydrogen are abstracted and form carbanion. The acidity of the carbonyl compounds depends on the stability of carbanion formed during the reaction. More stable the carbanion more acidic the alpha hydrogen. The acidities are measured in pka vale. Lower the pka value higher is the acidity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




