
In the depression of freezing point experiment, it is found that-
A) The vapour pressure of the solution is less than that of pure solvent.
B) The vapour pressure of solution is more than that of pure solvent.
C) Only solute molecules solidify at the freezing point.
D) Only solvent molecules solidify at the freezing point.
Answer
591.9k+ views
Hint: When non-volatile solute is added in solvent, a solution is formed. The vapour pressure of the solution decreases and the substance present in larger amounts solidifies when vapour pressure of solid becomes equal to the vapour pressure of liquid.
Complete step by step answer:
-When a non-volatile solute is added in a pure solvent, some solute molecules occupy the surface area along with molecules of pure solvent and the pure solvent’s tendency to go into vapour phase decreases due to which the vapour pressure of solution decreases. This means that the vapor pressure of solid and liquid solvent becomes equal at lower temperature. This is called the depression in the freezing point.
-Let us take example –
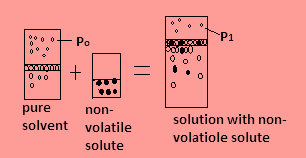
Suppose in the closed container, water is the pure solvent in the figure and its freezing point is \[{0^ \circ }{\text{C}}\] and water’s vapour pressure is suppose ${{\text{P}}_0}$ as evaporation at the surface of the water continues then-
1. When non-volatile solute is added in the water, the solution formed has vapor pressure ${{\text{P}}_1}$ which is less than the vapour pressure of the solvent as the number of particles in the solution increases due to solute and they occupy the surface area but they have less tendency to forms vapours.
2. The freezing point of solution becomes $ - {1^ \circ }{\text{C}}$ or less. This means the freezing point decreases when non-volatile solute is added. Since the molecules of solvent are more than that of solute so the molecules of solvent solidify at freezing point.
Hence, correct options are A and D.
Additional Information:
In the given graph, relation between vapour pressure and temperature is given for depression of freezing point-
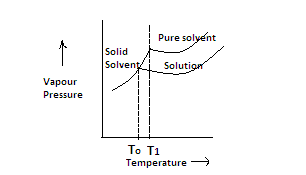
1. The pure solvent has more vapour pressure than the solution due to presence of non-volatile solute so its curve is drawn higher than the curve of solution. Now the vapors of solid solvent form a steep curve.
2. When the temperature at which the vapor pressure of solid solvent and liquid solvent equal becomes then it is called freezing point. This temperature is given by ${{\text{T}}_0}$.
3. When we draw the curve of solution to meet the curve of solid solvent we see that their vapor pressure becomes equal at lower temperature which is represented by ${{\text{T}}_1}$ .
4.The difference between these two temperatures is called depression in freezing point.
It is given as-
$\Delta {\text{T}} = {{\text{T}}_{\text{0}}}{\text{ - }}{{\text{T}}_{\text{1}}}$
Note:
Depression of freezing point is also given as-
$\Delta {\text{T = }}{{\text{K}}_f}{\text{m}}$ where m is the molality and ${{\text{K}}_f}$ is the cryoscopic constant which depends on the properties of solvent and solute. Molality is the number of moles of solutes dissolved in one kilogram solvent.
Complete step by step answer:
-When a non-volatile solute is added in a pure solvent, some solute molecules occupy the surface area along with molecules of pure solvent and the pure solvent’s tendency to go into vapour phase decreases due to which the vapour pressure of solution decreases. This means that the vapor pressure of solid and liquid solvent becomes equal at lower temperature. This is called the depression in the freezing point.
-Let us take example –

Suppose in the closed container, water is the pure solvent in the figure and its freezing point is \[{0^ \circ }{\text{C}}\] and water’s vapour pressure is suppose ${{\text{P}}_0}$ as evaporation at the surface of the water continues then-
1. When non-volatile solute is added in the water, the solution formed has vapor pressure ${{\text{P}}_1}$ which is less than the vapour pressure of the solvent as the number of particles in the solution increases due to solute and they occupy the surface area but they have less tendency to forms vapours.
2. The freezing point of solution becomes $ - {1^ \circ }{\text{C}}$ or less. This means the freezing point decreases when non-volatile solute is added. Since the molecules of solvent are more than that of solute so the molecules of solvent solidify at freezing point.
Hence, correct options are A and D.
Additional Information:
In the given graph, relation between vapour pressure and temperature is given for depression of freezing point-

1. The pure solvent has more vapour pressure than the solution due to presence of non-volatile solute so its curve is drawn higher than the curve of solution. Now the vapors of solid solvent form a steep curve.
2. When the temperature at which the vapor pressure of solid solvent and liquid solvent equal becomes then it is called freezing point. This temperature is given by ${{\text{T}}_0}$.
3. When we draw the curve of solution to meet the curve of solid solvent we see that their vapor pressure becomes equal at lower temperature which is represented by ${{\text{T}}_1}$ .
4.The difference between these two temperatures is called depression in freezing point.
It is given as-
$\Delta {\text{T}} = {{\text{T}}_{\text{0}}}{\text{ - }}{{\text{T}}_{\text{1}}}$
Note:
Depression of freezing point is also given as-
$\Delta {\text{T = }}{{\text{K}}_f}{\text{m}}$ where m is the molality and ${{\text{K}}_f}$ is the cryoscopic constant which depends on the properties of solvent and solute. Molality is the number of moles of solutes dissolved in one kilogram solvent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




