
In the compound between A and H, what type of bond is formed?
(A) Ionic
(B) Covalent
(C) Coordinate
(D) No bond is formed
Answer
580.8k+ views
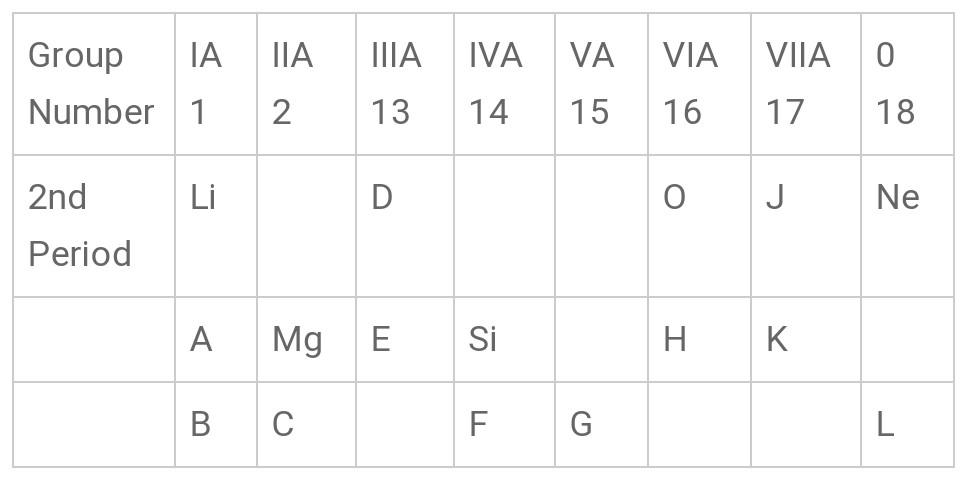
Hint: By looking in the below table, we would get an idea about which element is A and which is H. Determining their nature we can easily say what type of bond is formed between them.
Complete step by step solution:
From the table given above, we can see that A belongs to Group IA and H belongs to Group VIA. Since A is below Lithium (Li-Atomic No.3) and next Neon (Atomic No. 10), it must surely be Sodium (Na- Atomic No. 11). H is placed in Group VIA, before the potassium (K- Atomic No. 17), so it must be Sulphur (S) with Atomic Number 16. As we know Na is a metal and S is a Nonmetal, the bond formed between them must be an ionic bond and the compound is called Ionic compound.
Definition: Ionic bond is the force of attraction between a positive and negative ion. This bond will be formed when the metallic atom gives its electrons to the nonmetallic atom.
During this time metal will give its electrons to the nonmetal, in order to obtain the nearest stable electronic configuration. Similarly, nonmetal will also accept those electrons from the metals in order to obtain the nearest stable electronic configuration.
$2Na+S\to N{{a}_{2}}S$
Sodium metal by giving its electron will attain [Ne] configuration and Sulphur will obtain two electrons (one from each Na metal) to attain [Ar] configuration.
Therefore, the correct answer is option (A) Ionic.
Additional Information:
There are various other bonds. They are
- Covalent bond
- Coordinate bond
- Hydrogen bond
Note: Covalent bonds will be formed only between two nonmetals. Ionic bonds are formed between a metal and a nonmetal. Strength of ionic bonds depends on the difference of electronegativity of the bonded atoms.

Complete step by step solution:
From the table given above, we can see that A belongs to Group IA and H belongs to Group VIA. Since A is below Lithium (Li-Atomic No.3) and next Neon (Atomic No. 10), it must surely be Sodium (Na- Atomic No. 11). H is placed in Group VIA, before the potassium (K- Atomic No. 17), so it must be Sulphur (S) with Atomic Number 16. As we know Na is a metal and S is a Nonmetal, the bond formed between them must be an ionic bond and the compound is called Ionic compound.
Definition: Ionic bond is the force of attraction between a positive and negative ion. This bond will be formed when the metallic atom gives its electrons to the nonmetallic atom.
During this time metal will give its electrons to the nonmetal, in order to obtain the nearest stable electronic configuration. Similarly, nonmetal will also accept those electrons from the metals in order to obtain the nearest stable electronic configuration.
$2Na+S\to N{{a}_{2}}S$
Sodium metal by giving its electron will attain [Ne] configuration and Sulphur will obtain two electrons (one from each Na metal) to attain [Ar] configuration.
Therefore, the correct answer is option (A) Ionic.
Additional Information:
There are various other bonds. They are
- Covalent bond
- Coordinate bond
- Hydrogen bond
Note: Covalent bonds will be formed only between two nonmetals. Ionic bonds are formed between a metal and a nonmetal. Strength of ionic bonds depends on the difference of electronegativity of the bonded atoms.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




