
In Schottky defect:
A) An ion moves to interstitial position between the lattice points
B) Electrons are trapped in lattice sites
C) Some lattice sites are vacant
D) Some extra cations are present in interstitial spaces.
Answer
565.5k+ views
Hint:Schottky defect is a point defect and a type of vacancy defect.
This defect decreases the density of the solid.
Complete answer:
The question is asked that, from the four options given below which option suits best for the concept of Schottky defect.
For finding the answer, we should have a basic idea about the imperfections or defects in the solid lattice.
So now we can discuss some concepts on defects mainly on point defects.
Point defects- these defects are the irregularities around a point in the solid lattice i.e. the arrangement of the ions to generate an entire structure or it can be said as the deviation from the ideal arrangement around a point or atom in the lattice.
Point defects are mainly classified into three-
Stoichiometric defects
Non-stoichiometric defects
Impurity defects
These three types are again classified into many.
Stoichiometric defects are of two types -vacancy defects and interstitial defects.
Non-stoichiometric defects are classified into -metal excess and metal deficiency defects.
Metal excess defects are again classified to- metal excess defect due to anionic vacancies and metal excess defect due to presence of extra cations.
So now let’s see what are Schottky defects and some of its examples.
Schottky defect is a vacancy defect which comes under the point defect. So from referring it as point defect we know that the irregularity or the deviation from the ideal arrangement is around a point in the lattice or around an atom in the crystal lattice. In this defect, an atom or ions will be missing from the crystal lattice.
Hence the density of the solid will get decreased. The total number of ions or atoms that will be equal to the equal number of anions and cations.
For more clarity, if 6 lattice sites are vacant then it means that 3 cations and 3 anions are missing in the lattice.
Hence this type of defect maintains the electrical neutrality of the crystal.
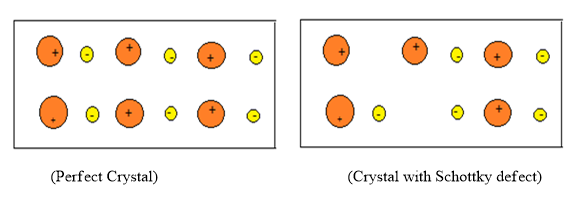
So the correct statement from the above given options is Option (C) .
Note:
Option A is correct for the Frenkel defect, in which the ions move into the interstitial site and hence the electrical neutrality is maintained as well as there is no change in the density of the solid.
Option B –refers to F-centre which is commonly seen with solids having the metal excess defect due to anionic vacancies.
Option D suits best for the non-stoichiometric metal excess defect, metal excess defect due to presence of extra cations.
This defect decreases the density of the solid.
Complete answer:
The question is asked that, from the four options given below which option suits best for the concept of Schottky defect.
For finding the answer, we should have a basic idea about the imperfections or defects in the solid lattice.
So now we can discuss some concepts on defects mainly on point defects.
Point defects- these defects are the irregularities around a point in the solid lattice i.e. the arrangement of the ions to generate an entire structure or it can be said as the deviation from the ideal arrangement around a point or atom in the lattice.
Point defects are mainly classified into three-
Stoichiometric defects
Non-stoichiometric defects
Impurity defects
These three types are again classified into many.
Stoichiometric defects are of two types -vacancy defects and interstitial defects.
Non-stoichiometric defects are classified into -metal excess and metal deficiency defects.
Metal excess defects are again classified to- metal excess defect due to anionic vacancies and metal excess defect due to presence of extra cations.
So now let’s see what are Schottky defects and some of its examples.
Schottky defect is a vacancy defect which comes under the point defect. So from referring it as point defect we know that the irregularity or the deviation from the ideal arrangement is around a point in the lattice or around an atom in the crystal lattice. In this defect, an atom or ions will be missing from the crystal lattice.
Hence the density of the solid will get decreased. The total number of ions or atoms that will be equal to the equal number of anions and cations.
For more clarity, if 6 lattice sites are vacant then it means that 3 cations and 3 anions are missing in the lattice.
Hence this type of defect maintains the electrical neutrality of the crystal.

So the correct statement from the above given options is Option (C) .
Note:
Option A is correct for the Frenkel defect, in which the ions move into the interstitial site and hence the electrical neutrality is maintained as well as there is no change in the density of the solid.
Option B –refers to F-centre which is commonly seen with solids having the metal excess defect due to anionic vacancies.
Option D suits best for the non-stoichiometric metal excess defect, metal excess defect due to presence of extra cations.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




