
In phosphorus atoms, find out the no. of paired electrons for l=1 and m=0.
A.3
B.1
C.2
D.0
Answer
521.9k+ views
Hint: A quantum number for an atomic orbital that describes its orbital angular momentum and tells about the shape of the orbital is known as azimuthal quantum number. We can also call it an orbital angular momentum quantum number, orbital quantum number or second quantum number. It is represented by the letter l. We can predict the number of paired electrons using the orbital, which is predicted using the azimuthal quantum number.
Complete step by step answer:
We know that phosphorus is a chemical element that has a symbol P. The atomic number of phosphorus is fifteen. Phosphorus is a non-metal and it belongs to group 15 and period 3. It is a p-block element.
The electrons per shell in phosphorus is 2,8,5.
We can express the electronic configuration of phosphorus as $1{s^2}2{s^2}2{p^6}3{s^2}3{p^3}$.
We have to know that if l=1, the value of azimuthal quantum number is one.
So, we have to know that
If l=0, the orbital is s-orbital and the shape is spherical.
If l=1, the orbital is p-orbital and the shape is dumb-bell.
If l=2, the orbital is d-orbital and the shape is clover leaf.
If l=3, the orbital is f-orbital.
The subshells that contain l=1 in the electronic configuration are 2p and 3p.
Each sub-shell contains 3 orbitals that have ${m_l}$ and have -1,0,+1.
Therefore, the orbitals that have l=1 and m=0 are the second orbitals present in the p-subshell.
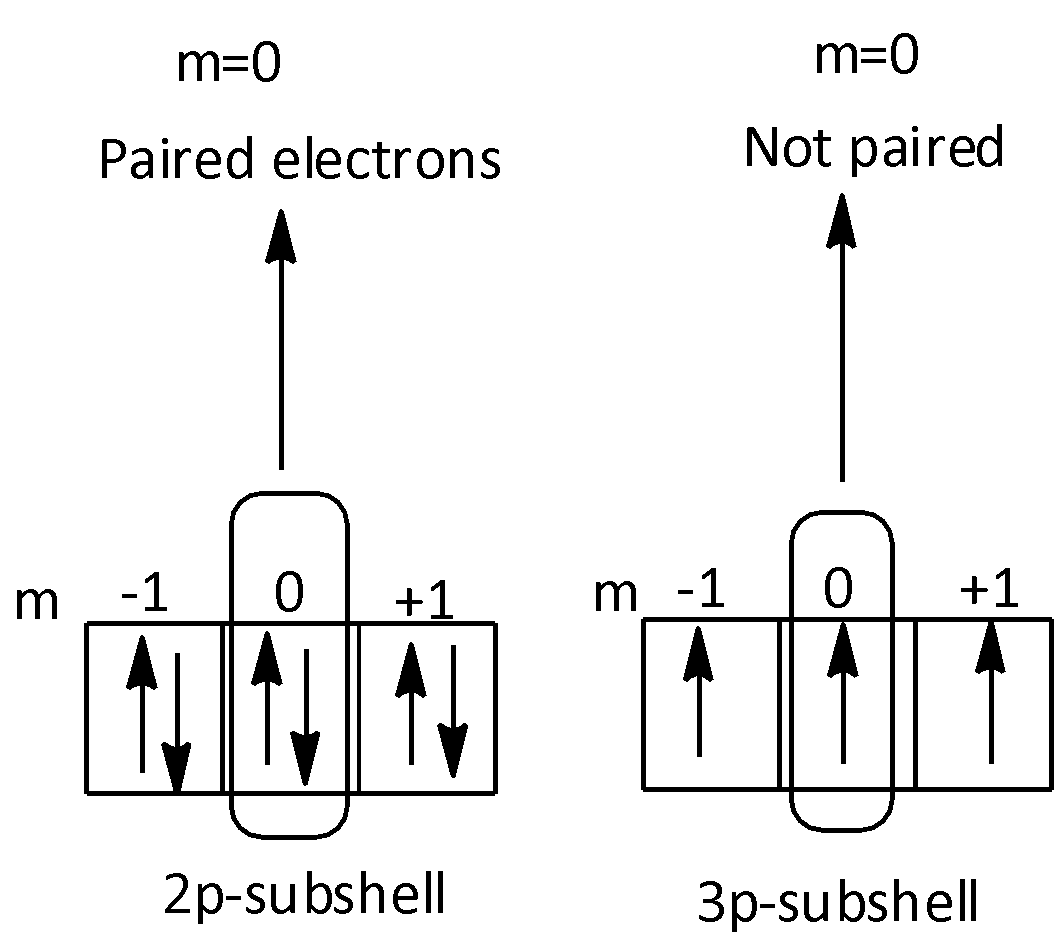
The number of paired electrons which would fit to second orbitals of p-subshell of n=2 and n=3 are two.
So, the correct answer is Option C.
Note:
We have to know that elemental phosphorus is found in two major forms, white phosphorus and red phosphorus. Due to its high reactive nature, phosphorus is not found as a free element on Earth. Phosphorus along with nitrogen, arsenic, antimony, and bismuth, are classified as pnictogen. A majority of the phosphorus compounds are used as fertilizers.
Complete step by step answer:
We know that phosphorus is a chemical element that has a symbol P. The atomic number of phosphorus is fifteen. Phosphorus is a non-metal and it belongs to group 15 and period 3. It is a p-block element.
The electrons per shell in phosphorus is 2,8,5.
We can express the electronic configuration of phosphorus as $1{s^2}2{s^2}2{p^6}3{s^2}3{p^3}$.
We have to know that if l=1, the value of azimuthal quantum number is one.
So, we have to know that
If l=0, the orbital is s-orbital and the shape is spherical.
If l=1, the orbital is p-orbital and the shape is dumb-bell.
If l=2, the orbital is d-orbital and the shape is clover leaf.
If l=3, the orbital is f-orbital.
The subshells that contain l=1 in the electronic configuration are 2p and 3p.
Each sub-shell contains 3 orbitals that have ${m_l}$ and have -1,0,+1.
Therefore, the orbitals that have l=1 and m=0 are the second orbitals present in the p-subshell.

The number of paired electrons which would fit to second orbitals of p-subshell of n=2 and n=3 are two.
So, the correct answer is Option C.
Note:
We have to know that elemental phosphorus is found in two major forms, white phosphorus and red phosphorus. Due to its high reactive nature, phosphorus is not found as a free element on Earth. Phosphorus along with nitrogen, arsenic, antimony, and bismuth, are classified as pnictogen. A majority of the phosphorus compounds are used as fertilizers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




